当前位置:
X-MOL 学术
›
Prog. Nucl. Magn. Reson. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isotope Effects on Chemical Shifts in the Study of Hydrogen Bonded Biological Systems
Progress in Nuclear Magnetic Resonance Spectroscopy ( IF 6.1 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.pnmrs.2020.08.001 Poul Erik Hansen 1
Progress in Nuclear Magnetic Resonance Spectroscopy ( IF 6.1 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.pnmrs.2020.08.001 Poul Erik Hansen 1
Affiliation
|
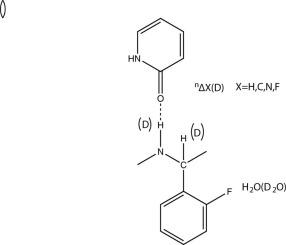
This review deals with biological systems and with deuterium isotope effects on chemical shifts caused by the replacement of OH, NH or SH protons by deuterons. Hydrogen bonding is clearly of central importance. Isotope effects on chemical shifts seems very suitable for use in studies of structures and reactions in the interior of proteins, as exchange of the label can be expected to be slow. One-bond deuterium isotope effects on 15N chemical shifts, and two-bond effects on 1H chemical shifts for N(D)Hx systems can be used to gauge hydrogen bond strength in proteins as well as in salt bridges. Solvent isotope effects on 19F chemical shifts show promise in monitoring solvent access. Equilibrium isotope effects need in some cases to be taken into account. Schemes for calculation of deuterium isotope effects on chemical shifts are discussed and it is demonstrated how calculations may be used in the study of complex biological systems.
中文翻译:

同位素对氢键生物系统研究中化学位移的影响
本综述涉及生物系统和氘同位素对由氘核替代 OH、NH 或 SH 质子引起的化学位移的影响。氢键显然是至关重要的。同位素对化学位移的影响似乎非常适合用于研究蛋白质内部的结构和反应,因为预计标记的交换会很慢。对于 N(D)Hx 系统,单键氘同位素对 15N 化学位移的影响和对 1H 化学位移的双键效应可用于衡量蛋白质和盐桥中的氢键强度。溶剂同位素对 19F 化学位移的影响显示出监测溶剂进入的前景。在某些情况下需要考虑平衡同位素效应。
更新日期:2020-10-01
中文翻译:

同位素对氢键生物系统研究中化学位移的影响
本综述涉及生物系统和氘同位素对由氘核替代 OH、NH 或 SH 质子引起的化学位移的影响。氢键显然是至关重要的。同位素对化学位移的影响似乎非常适合用于研究蛋白质内部的结构和反应,因为预计标记的交换会很慢。对于 N(D)Hx 系统,单键氘同位素对 15N 化学位移的影响和对 1H 化学位移的双键效应可用于衡量蛋白质和盐桥中的氢键强度。溶剂同位素对 19F 化学位移的影响显示出监测溶剂进入的前景。在某些情况下需要考虑平衡同位素效应。
































 京公网安备 11010802027423号
京公网安备 11010802027423号