Solid State Nuclear Magnetic Resonance ( IF 3.2 ) Pub Date : 2022-11-04 , DOI: 10.1016/j.ssnmr.2022.101837 Sean T Holmes 1 , Cameron S Vojvodin 1 , Natan Veinberg 2 , Emilia M Iacobelli 2 , David A Hirsh 2 , Robert W Schurko 1
|
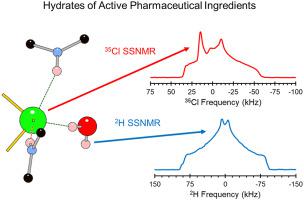
This study uses 35Cl and 2H solid-state NMR (SSNMR) spectroscopy and dispersion-corrected plane-wave density functional theory (DFT) calculations to characterize the molecular-level structures and dynamics of hydrates of active pharmaceutical ingredients (APIs). We use 35Cl SSNMR to measure the EFG tensors of the chloride ions to characterize hydrated forms of hydrochloride salts of APIs, along with two corresponding anhydrous forms. DFT calculations are used to refine the crystal structures of the APIs and determine relationships between the 35Cl EFG tensors and the spatial arrangements of proximate hydrogen bonds, which are particularly influenced by interactions with water molecules. We find that the relationship between 35Cl EFG tensors and local hydrogen bonding geometries is complex, but meaningful structure/property relationships can be garnered through use of DFT calculations. Specifically, for every case in which such a comparison could be made, we find that the hydrate has a smaller magnitude of CQ than the corresponding anhydrous form, indicating a chloride ion environment with a ground-state electron density of higher spherical symmetry in the former. Finally, variable-temperature 35Cl and 2H SSNMR experiments on a deuterium-exchanged sample of the API cimetidine hydrochloride monohydrate are used to monitor temperature-dependent influences on the spectra that may arise from motional influences on the 35Cl and 2H EFG tensors. From the 2H SSNMR spectra, we determine that the motions of water molecules are characterized by jump-like motions about their C2 rotational axes that occur on timescales that are unlikely to influence the 35Cl central-transition (+1/2 ↔︎ −1/2) powder patterns (this is confirmed by 35Cl SSNMR). Together, these methods show great promise for the future study of APIs in their bulk and dosage forms, especially variable hydrates in which crystallographic water content varies with external conditions such as humidity.
中文翻译:

活性药物成分的水合物:35Cl 和 2H 固态 NMR 和 DFT 研究
本研究使用35 Cl 和2 H 固态核磁共振 (SSNMR) 光谱和色散校正平面波密度泛函理论 (DFT) 计算来表征活性药物成分 (API) 水合物的分子水平结构和动力学。我们使用35 Cl SSNMR 测量氯离子的 EFG 张量,以表征 API 盐酸盐的水合形式以及两种相应的无水形式。DFT 计算用于细化 API 的晶体结构,并确定35 Cl EFG 张量与邻近氢键空间排列之间的关系,这尤其受与水分子相互作用的影响。我们发现之间的关系35 Cl EFG 张量和局部氢键几何结构很复杂,但可以通过使用 DFT 计算获得有意义的结构/性质关系。具体来说,对于可以进行这种比较的每种情况,我们发现水合物的C Q幅度小于相应的无水形式,表明氯离子环境具有更高球对称的基态电子密度以前的。最后,在 API 西咪替丁盐酸盐一水合物的氘交换样品上进行的变温35 Cl 和2 H SSNMR 实验用于监测可能由35 Cl 和35 Cl的运动影响引起的对光谱的温度依赖性影响。2 H EFG 张量。从2 H SSNMR 光谱,我们确定水分子运动的特征是围绕其C 2旋转轴的跳跃式运动,这种运动发生在不太可能影响35 Cl 中心跃迁 (+1/2 ↔︎ −)的时间尺度上1/2) 粉末图案(这由35 Cl SSNMR 证实)。总之,这些方法为未来研究散装和剂型的 APIs,特别是结晶水含量随外部条件(如湿度)而变化的可变水合物显示出巨大的希望。
































 京公网安备 11010802027423号
京公网安备 11010802027423号