当前位置:
X-MOL 学术
›
Solid State Nucl. Magn. Reson.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbon-detected deuterium solid-state NMR rotating frame relaxation measurements for protein methyl groups under magic angle spinning
Solid State Nuclear Magnetic Resonance ( IF 3.2 ) Pub Date : 2024-02-22 , DOI: 10.1016/j.ssnmr.2024.101922 Liliya Vugmeyster , Dmitry Ostrovsky , Riqiang Fu
Solid State Nuclear Magnetic Resonance ( IF 3.2 ) Pub Date : 2024-02-22 , DOI: 10.1016/j.ssnmr.2024.101922 Liliya Vugmeyster , Dmitry Ostrovsky , Riqiang Fu
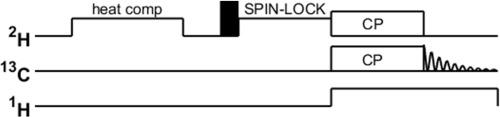
|
Deuterium rotating frame solid-state NMR relaxation measurements (H ) are important tools in quantitative studies of molecular dynamics. We demonstrate how H to C cross-polarization (CP) approaches under 10–40 kHz magic angle spinning rates can be combined with the H blocks to allow for extension of deuterium rotating frame relaxation studies to methyl groups in biomolecules. This extension permits detection on the C nuclei and, hence, for the achievement of site-specific resolution. The measurements are demonstrated using a nine-residue low complexity peptide with the sequence GGKGMGFGL, in which a single selective −CD label is placed at the methionine residue. Carbon-detected measurements are compared with the deuterium direct-detection results, which allows for fine-tuning of experimental approaches. In particular, we show how the adiabatic respiration CP scheme and the double adiabatic sweep on the H and C channels can be combined with the H relaxation rates measurement. Off-resonance H measurements are investigated in addition to the on-resonance condition, as they extent the range of effective spin-locking field.
中文翻译:

魔角旋转下蛋白质甲基的碳检测氘固态核磁共振旋转框架弛豫测量
氘旋转框架固态核磁共振弛豫测量 (H ) 是分子动力学定量研究的重要工具。我们演示了如何在 10-40 kHz 魔角旋转速率下实现 H 到 C 交叉极化 (CP) 与 H 块的结合,从而将氘旋转框架弛豫研究扩展到生物分子中的甲基。该扩展允许对 C 核进行检测,从而实现位点特异性分辨率。使用具有序列 GGKGMGFGL 的九残基低复杂性肽来证明测量结果,其中在甲硫氨酸残基处放置单个选择性 -CD 标签。将碳检测测量结果与氘直接检测结果进行比较,从而可以对实验方法进行微调。特别是,我们展示了如何将绝热呼吸 CP 方案以及 H 和 C 通道上的双绝热扫描与 H 弛豫率测量相结合。除了共振条件外,还研究了非共振 H 测量,因为它们扩大了有效自旋锁定场的范围。
更新日期:2024-02-22
中文翻译:

魔角旋转下蛋白质甲基的碳检测氘固态核磁共振旋转框架弛豫测量
氘旋转框架固态核磁共振弛豫测量 (H ) 是分子动力学定量研究的重要工具。我们演示了如何在 10-40 kHz 魔角旋转速率下实现 H 到 C 交叉极化 (CP) 与 H 块的结合,从而将氘旋转框架弛豫研究扩展到生物分子中的甲基。该扩展允许对 C 核进行检测,从而实现位点特异性分辨率。使用具有序列 GGKGMGFGL 的九残基低复杂性肽来证明测量结果,其中在甲硫氨酸残基处放置单个选择性 -CD 标签。将碳检测测量结果与氘直接检测结果进行比较,从而可以对实验方法进行微调。特别是,我们展示了如何将绝热呼吸 CP 方案以及 H 和 C 通道上的双绝热扫描与 H 弛豫率测量相结合。除了共振条件外,还研究了非共振 H 测量,因为它们扩大了有效自旋锁定场的范围。































 京公网安备 11010802027423号
京公网安备 11010802027423号