当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Amphiphilic electrolyte additive as an ion-flow stabilizer enables superb zinc metal batteries
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4ee00318g Zimin Yang 1, 2 , Yilun Sun 1 , Siting Deng 1, 3 , Hao Tong 1, 2 , Mingqiang Wu 1, 3 , Xinbin Nie 4 , Yifan Su 4 , Guanjie He 5 , Yinghe Zhang 6 , Jianwei Li 4 , Guoliang Chai 1, 7
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4ee00318g Zimin Yang 1, 2 , Yilun Sun 1 , Siting Deng 1, 3 , Hao Tong 1, 2 , Mingqiang Wu 1, 3 , Xinbin Nie 4 , Yifan Su 4 , Guanjie He 5 , Yinghe Zhang 6 , Jianwei Li 4 , Guoliang Chai 1, 7
Affiliation
|
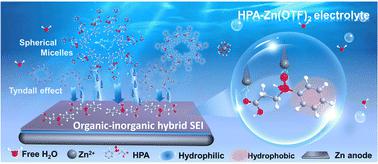
Irreversible Zn plating/stripping along with interfacial degradation seriously affect the practical applications of aqueous zinc-ion batteries. Herein, 3-(hydroxy(phenyl)phosphoryl)propanoic acid (HPA) is introduced as an electrolyte additive that constructs a spherical micellar molecular network via association of amphiphilic groups and multiple coordination sites to directionally adsorb/transfer Zn2+ in aqueous electrolyte, thus serving as an ion-flow stabilizer. Moreover, the strong adsorption between HPA and the zinc surface induces the formation of an in situ organic–inorganic hybrid solid electrolyte interphase layer, which further promotes the charge transfer kinetics and suppresses interfacial parasitic reactions. As a result, an ultra-high average Zn plating/stripping efficiency of 99.91% over 2100 cycles at 4 mA cm−2 is achieved. Additionally, the symmetrical cell with HPA exhibits outstanding reversibility at an unprecedentedly high current density of 120 mA cm−2. Surprisingly, the initial coulombic efficiency of Zn//Cu cell is 71.74% after 7-day calendar aging, which is better than a cell without HPA (42.59%). Furthermore, the Zn//MnO2 cell exhibits superior capacity retention of 80% after 1100 cycles at 2 A g−1 compared to the cell without HPA (37%). This study provides an in-depth insight into understanding the molecular network regulation of aqueous-based electrolytes, thus shedding light on a universal approach toward ultra-stable battery applications.
中文翻译:

作为离子流稳定剂的两亲电解质添加剂可实现卓越的锌金属电池
不可逆的镀锌/剥离以及界面退化严重影响水系锌离子电池的实际应用。本文引入3-(羟基(苯基)磷酰基)丙酸(HPA)作为电解液添加剂,通过两亲基团和多个配位位点的缔合构建球形胶束分子网络,从而在水性电解液中定向吸附/转移Zn 2+,从而充当离子流稳定器。此外,HPA和锌表面之间的强吸附导致原位有机-无机杂化固体电解质界面层的形成,进一步促进电荷转移动力学并抑制界面寄生反应。结果,在4 mA cm -2下经过2100次循环,实现了99.91%的超高平均镀锌/剥离效率。此外,具有HPA的对称电池在120 mA cm -2的前所未有的高电流密度下表现出出色的可逆性。令人惊讶的是,经过7天日历老化后,Zn//Cu电池的初始库伦效率为71.74%,优于没有HPA的电池(42.59%)。此外,与没有HPA的电池(37%)相比,Zn//MnO 2电池在2 A g -1 1100次循环后表现出优异的80%的容量保持率。这项研究深入了解了水基电解质的分子网络调节,从而揭示了超稳定电池应用的通用方法。
更新日期:2024-04-16
中文翻译:

作为离子流稳定剂的两亲电解质添加剂可实现卓越的锌金属电池
不可逆的镀锌/剥离以及界面退化严重影响水系锌离子电池的实际应用。本文引入3-(羟基(苯基)磷酰基)丙酸(HPA)作为电解液添加剂,通过两亲基团和多个配位位点的缔合构建球形胶束分子网络,从而在水性电解液中定向吸附/转移Zn 2+,从而充当离子流稳定器。此外,HPA和锌表面之间的强吸附导致原位有机-无机杂化固体电解质界面层的形成,进一步促进电荷转移动力学并抑制界面寄生反应。结果,在4 mA cm -2下经过2100次循环,实现了99.91%的超高平均镀锌/剥离效率。此外,具有HPA的对称电池在120 mA cm -2的前所未有的高电流密度下表现出出色的可逆性。令人惊讶的是,经过7天日历老化后,Zn//Cu电池的初始库伦效率为71.74%,优于没有HPA的电池(42.59%)。此外,与没有HPA的电池(37%)相比,Zn//MnO 2电池在2 A g -1 1100次循环后表现出优异的80%的容量保持率。这项研究深入了解了水基电解质的分子网络调节,从而揭示了超稳定电池应用的通用方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号