Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Steady-State and transient kinetic investigations of the oxidation of NO over Pt/SiO2
Journal of Catalysis ( IF 7.3 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.jcat.2024.115483 Moses Mawanga , Jia Yang , Edd A. Blekkan
Journal of Catalysis ( IF 7.3 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.jcat.2024.115483 Moses Mawanga , Jia Yang , Edd A. Blekkan
|
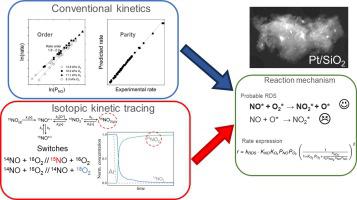
The reaction mechanism of the oxidation of NO under conditions of high NO concentration over a 2 wt% Pt/SiO was studied using both kinetic and transient isotopic tracing experiments. The reaction mechanism was found to involve both NO adsorption and oxygen adsorption, contrary to the situation under low NO concentration where NO reacts from the gas phase. The kinetic rate data could be fitted to the Langmuir-Hinshelwood model permitting the estimation of rate and equilibrium constants of elementary steps. These steps were investigated using transient responses following independent reactant gas isotopic switches for tracing the nitrogen path and the oxygen path during NO oxidation. These transients could be sufficiently described by a microkinetic model based on two pools of NO intermediates, one pool each for the adsorbed molecular oxygen and atomically adsorbed oxygen. Surface atomic oxygen was found to be formed by two different routes: via a direct dissociation of molecularly adsorbed oxygen and also via an assisted pathway that leads to NO formation. The latter surface reaction between adsorbed NO and adsorbed molecular oxygen comprised the kinetically relevant step. Insights from the two experimental and model approaches were in good agreement and provided a coherent reaction mechanism for NO oxidation under high NO reactant concentration.
中文翻译:

Pt/SiO2 上 NO 氧化的稳态和瞬态动力学研究
采用动力学和瞬态同位素示踪实验研究了 2 wt% Pt/SiO2 上高 NO 浓度条件下 NO 氧化的反应机理。研究发现反应机理涉及NO吸附和氧吸附,这与低NO浓度下NO从气相发生反应的情况相反。动力学速率数据可以适合 Langmuir-Hinshelwood 模型,从而可以估计基本步骤的速率和平衡常数。使用独立反应气体同位素开关后的瞬态响应来研究这些步骤,以追踪 NO 氧化过程中的氮气路径和氧气路径。这些瞬态可以通过基于两个 NO 中间体池的微动力学模型来充分描述,其中每个池用于吸附分子氧和原子吸附氧。研究发现表面原子氧是通过两种不同的途径形成的:通过分子吸附氧的直接解离,以及通过导致NO形成的辅助途径。吸附的 NO 和吸附的分子氧之间的后者表面反应包括动力学相关的步骤。两种实验和模型方法的见解非常一致,并为高 NO 反应物浓度下的 NO 氧化提供了连贯的反应机制。
更新日期:2024-04-09
中文翻译:

Pt/SiO2 上 NO 氧化的稳态和瞬态动力学研究
采用动力学和瞬态同位素示踪实验研究了 2 wt% Pt/SiO2 上高 NO 浓度条件下 NO 氧化的反应机理。研究发现反应机理涉及NO吸附和氧吸附,这与低NO浓度下NO从气相发生反应的情况相反。动力学速率数据可以适合 Langmuir-Hinshelwood 模型,从而可以估计基本步骤的速率和平衡常数。使用独立反应气体同位素开关后的瞬态响应来研究这些步骤,以追踪 NO 氧化过程中的氮气路径和氧气路径。这些瞬态可以通过基于两个 NO 中间体池的微动力学模型来充分描述,其中每个池用于吸附分子氧和原子吸附氧。研究发现表面原子氧是通过两种不同的途径形成的:通过分子吸附氧的直接解离,以及通过导致NO形成的辅助途径。吸附的 NO 和吸附的分子氧之间的后者表面反应包括动力学相关的步骤。两种实验和模型方法的见解非常一致,并为高 NO 反应物浓度下的 NO 氧化提供了连贯的反应机制。
































 京公网安备 11010802027423号
京公网安备 11010802027423号