当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly selective electrocatalytic reduction of CO2 to ethane over a petal-like Zn(OH)2/Cu2+1O/Cu foam catalyst at low overpotentials
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2024-05-02 , DOI: 10.1039/d4ta00502c Hui-Hui Cao 1 , Zhen-Hong He 1 , Yue Tian 1 , Yue-Xia Yang 1 , Xin Wang 1 , Kuan Wang 1 , Weitao Wang 1 , Huan Wang 1 , Jiajie Liu 1 , Zhao-Tie Liu 1, 2
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2024-05-02 , DOI: 10.1039/d4ta00502c Hui-Hui Cao 1 , Zhen-Hong He 1 , Yue Tian 1 , Yue-Xia Yang 1 , Xin Wang 1 , Kuan Wang 1 , Weitao Wang 1 , Huan Wang 1 , Jiajie Liu 1 , Zhao-Tie Liu 1, 2
Affiliation
|
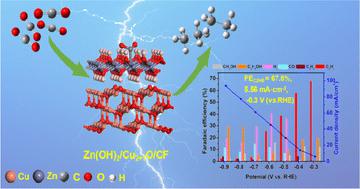
The electrocatalytic CO2 reduction reaction (ECO2RR) is a crucial process for converting CO2 into value-added chemicals. Achieving high efficiency in the synthesis of ethane through the ECO2RR remains a challenging task. In this work, we developed a Cu2+1O species combined with Zn(OH)2 loaded on a copper foam catalyst, denoted as Zn(OH)2/Cu2+1O/CF, presenting a lamellar structure, which offers high selectivity to ethane at low overpotentials. Typically, at −0.3 V (vs. RHE), the catalyst showed high activity, excellent stability, and a high ethane faradaic efficiency(FEC2H6) of 67.8% with a current density of 5.56 mA cm−2 in 0.1 M KCl electrolyte. The FEC2H6 still reached 25.4% with a higher current density of 29.40 mA cm−2 at −0.5 V (vs. RHE). These values are much higher than those of state-of-the-art catalysts for ethane formation at low overpotentials. The catalytic performances were derived from the metal defects in Cu2+1O offering abundant Cu+ and Cu2+ valent species, which could be stabilized by the Zn(OH)2. They achieved excellent stability in a long-term operation for three days. The catalytic performances and catalyst's structure were systemically investigated via diverse characterization techniques, and the formation of ethane involved *CO, *COOH, and *CO–*CH2 intermediates, which were confirmed by using in situ Raman tests and DFT calculations. This work offers an essential reference in designing and constructing efficient Cu-based catalysts with stable Cu+ and Cu2+ valences, especially in reactions such as electrolytic CO2 reduction.
中文翻译:

花瓣状 Zn(OH)2/Cu2+1O/Cu 泡沫催化剂在低过电势下高选择性电催化还原 CO2 为乙烷
电催化CO 2还原反应(ECO 2 RR)是将CO 2转化为增值化学品的关键过程。通过 ECO 2 RR实现乙烷合成的高效率仍然是一项具有挑战性的任务。在这项工作中,我们开发了一种与Zn(OH) 2负载在泡沫铜催化剂上的Cu 2+1 O物种,表示为Zn(OH) 2 /Cu 2+1 O/CF,呈现层状结构,这提供了在低过电势下对乙烷具有高选择性。通常,在-0.3 V(相对于RHE)下,催化剂表现出高活性、优异的稳定性以及67.8%的高乙烷法拉第效率(FE C 2 H 6 ) ,0.1 M中的电流密度为5.56 mA cm -2氯化钾电解质。在-0.5 V(相对于RHE)下,FE C 2 H 6仍达到25.4%,电流密度更高,为29.40 mA cm -2 。这些值远高于在低过电势下形成乙烷的最先进催化剂的值。催化性能源自Cu 2+1 O中的金属缺陷提供丰富的Cu +和Cu 2+价态物质,其可以被Zn(OH) 2稳定。他们在三天的长期运行中取得了出色的稳定性。通过多种表征技术系统地研究了催化性能和催化剂结构,乙烷的形成涉及*CO、*COOH和*CO–*CH 2中间体,并通过原位拉曼测试和DFT计算证实了这一点。这项工作为设计和构建具有稳定Cu +和Cu 2+价态的高效铜基催化剂,特别是在电解CO 2还原等反应中提供了重要的参考。
更新日期:2024-05-02
中文翻译:

花瓣状 Zn(OH)2/Cu2+1O/Cu 泡沫催化剂在低过电势下高选择性电催化还原 CO2 为乙烷
电催化CO 2还原反应(ECO 2 RR)是将CO 2转化为增值化学品的关键过程。通过 ECO 2 RR实现乙烷合成的高效率仍然是一项具有挑战性的任务。在这项工作中,我们开发了一种与Zn(OH) 2负载在泡沫铜催化剂上的Cu 2+1 O物种,表示为Zn(OH) 2 /Cu 2+1 O/CF,呈现层状结构,这提供了在低过电势下对乙烷具有高选择性。通常,在-0.3 V(相对于RHE)下,催化剂表现出高活性、优异的稳定性以及67.8%的高乙烷法拉第效率(FE C 2 H 6 ) ,0.1 M中的电流密度为5.56 mA cm -2氯化钾电解质。在-0.5 V(相对于RHE)下,FE C 2 H 6仍达到25.4%,电流密度更高,为29.40 mA cm -2 。这些值远高于在低过电势下形成乙烷的最先进催化剂的值。催化性能源自Cu 2+1 O中的金属缺陷提供丰富的Cu +和Cu 2+价态物质,其可以被Zn(OH) 2稳定。他们在三天的长期运行中取得了出色的稳定性。通过多种表征技术系统地研究了催化性能和催化剂结构,乙烷的形成涉及*CO、*COOH和*CO–*CH 2中间体,并通过原位拉曼测试和DFT计算证实了这一点。这项工作为设计和构建具有稳定Cu +和Cu 2+价态的高效铜基催化剂,特别是在电解CO 2还原等反应中提供了重要的参考。































 京公网安备 11010802027423号
京公网安备 11010802027423号