当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulating Alcohol Adsorption Modes for Boosting Electrooxidation-Assisted Hydrogen Production
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-05-02 , DOI: 10.1021/acscatal.4c01078 Pei Zhu 1 , Yongli Shen 1 , Zhi-Ming Zhang 1 , Dingsheng Wang 2 , Baojuan Xi 3 , Xuguang An 4 , Shenglin Xiong 3 , Changhua An 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-05-02 , DOI: 10.1021/acscatal.4c01078 Pei Zhu 1 , Yongli Shen 1 , Zhi-Ming Zhang 1 , Dingsheng Wang 2 , Baojuan Xi 3 , Xuguang An 4 , Shenglin Xiong 3 , Changhua An 1
Affiliation
|
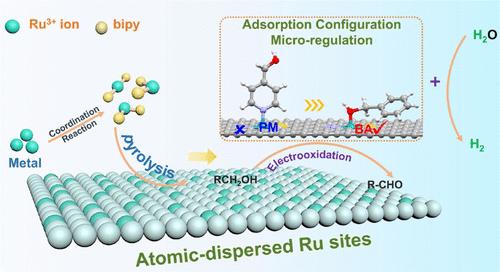
Oxygen evolution reaction (OER) suffers from sluggish kinetics and results in the increasing cost of hydrogen production. The exploration of an appropriate anode organic reaction occurring at low potential represents a feasible strategy to accelerate the implementation of water splitting in practice. Herein, we develop a ligand-confining thermolysis strategy to fabricate a Ru single-atom catalyst (Ru-SA/NSC) on N,S-codoped carbon. The adsorption mode effects of substrate alcohols on the electrocatalytic oxidation of Ru-SA/NSC are unraveled through modulation of substituent groups. The horizontal adsorption through the O atom on Ru-SA/NSC significantly facilitates the benzyl alcohol oxidation, delivering ultralow potential of 0.97 V vs reversible hydrogen electrode (RHE) at 10 mA cm–2 with high yield (∼96%), selectivity (∼99%), and Faraday efficiency (∼100%) to produce aldehydes. The vertical adsorption through the N atom in pyridine methanol over Ru-SA/NSC has no response to the reaction. Furthermore, in the coupling device of alcohol oxidation and hydrogen evolution reaction, hydrogen production with a low potential of 1.21 V at 10 mA cm–2 is achieved, surpassing that of benchmark Pt/C||IrO2 (1.56 V) and the state-of-the-art reports. This study provides insights into the design of nanocatalysts toward the rational conversion of organic molecules to value-added chemicals and concurrently produces clean energy carriers.
中文翻译:

调节醇吸附模式以促进电氧化辅助产氢
析氧反应(OER)动力学缓慢,导致氢气生产成本增加。探索在低电势下发生适当的阳极有机反应是加速水分解在实践中实施的可行策略。在此,我们开发了一种配体限制热解策略,在 N,S 共掺杂碳上制备 Ru 单原子催化剂 (Ru-SA/NSC)。通过取代基的调节揭示了底物醇对 Ru-SA/NSC 电催化氧化的吸附模式影响。 Ru-SA/NSC 上 O 原子的水平吸附显着促进了苯甲醇的氧化,在 10 mA cm –2 电流下相对于可逆氢电极 (RHE) 提供 0.97 V 的超低电势–2,并具有高产率 (∼96 %)、选择性(∼99%)和法拉第效率(∼100%)来生产醛。 Ru-SA/NSC 上吡啶甲醇中 N 原子的垂直吸附对反应没有响应。此外,在醇氧化与析氢反应耦合装置中,在10 mA cm –2 下实现了1.21 V的低电势产氢,超越了基准Pt/C||IrO 2 (1.56 V) 和最先进的报告。这项研究为纳米催化剂的设计提供了见解,以将有机分子合理转化为增值化学品,同时产生清洁能源载体。
更新日期:2024-05-02
中文翻译:

调节醇吸附模式以促进电氧化辅助产氢
析氧反应(OER)动力学缓慢,导致氢气生产成本增加。探索在低电势下发生适当的阳极有机反应是加速水分解在实践中实施的可行策略。在此,我们开发了一种配体限制热解策略,在 N,S 共掺杂碳上制备 Ru 单原子催化剂 (Ru-SA/NSC)。通过取代基的调节揭示了底物醇对 Ru-SA/NSC 电催化氧化的吸附模式影响。 Ru-SA/NSC 上 O 原子的水平吸附显着促进了苯甲醇的氧化,在 10 mA cm –2 电流下相对于可逆氢电极 (RHE) 提供 0.97 V 的超低电势–2,并具有高产率 (∼96 %)、选择性(∼99%)和法拉第效率(∼100%)来生产醛。 Ru-SA/NSC 上吡啶甲醇中 N 原子的垂直吸附对反应没有响应。此外,在醇氧化与析氢反应耦合装置中,在10 mA cm –2 下实现了1.21 V的低电势产氢,超越了基准Pt/C||IrO 2 (1.56 V) 和最先进的报告。这项研究为纳米催化剂的设计提供了见解,以将有机分子合理转化为增值化学品,同时产生清洁能源载体。
































 京公网安备 11010802027423号
京公网安备 11010802027423号