当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interfacial Degradation Mechanism of Nanostructured LiCoO2 for Li6PS5Cl-Based All-Solid-State Batteries
Chemistry of Materials ( IF 8.6 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.chemmater.4c00629 Kanghyeon Kim 1 , Seunggoo Jun 2 , Taehun Kim 1 , Jong Seok Kim 2 , Seonghyun Lee 1 , Gawon Song 1 , Junsung Park 1 , Yoon Seok Jung 2 , Kyu Tae Lee 1
Chemistry of Materials ( IF 8.6 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.chemmater.4c00629 Kanghyeon Kim 1 , Seunggoo Jun 2 , Taehun Kim 1 , Jong Seok Kim 2 , Seonghyun Lee 1 , Gawon Song 1 , Junsung Park 1 , Yoon Seok Jung 2 , Kyu Tae Lee 1
Affiliation
|
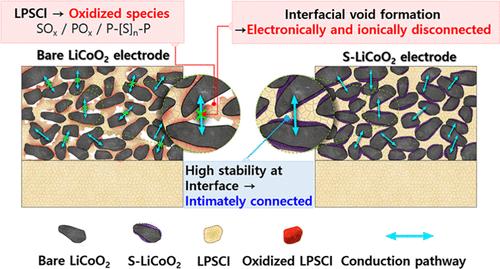
Interfacial degradation of Li6PS5Cl (LPSCl) with oxide cathode materials during cycling, particularly the formation of interfacial voids, leads to poor electrochemical performance. The formation of these voids is driven by two distinct mechanisms: the volumetric changes of oxide cathode materials during cycling and the volumetric shrinkage of LPSCl due to oxidative decomposition. However, the relative contribution of each route to void formation remains ambiguous, especially for nanostructured cathode materials. This study highlights the predominant influence of oxidative decomposition of LPSCl on the nanostructured LiCoO2 surface in the formation of interfacial voids when compared to the volumetric changes of LiCoO2 between charging and discharging. The interfacial degradation behavior is compared between bare LiCoO2 and LiCoO2–Li2SnO3 core–shell nanoparticles. Both types of nanoparticles exhibit comparable absolute volume changes of LiCoO2 during cycling, due to their similar particle sizes and reversible capacities, effectively ruling out the impact of volumetric changes of LiCoO2 on void formation. However, LiCoO2–Li2SnO3 shows mitigated interfacial void formation compared to bare LiCoO2, resulting in improved electrochemical performance. This is attributed to the fact that LiCoO2–Li2SnO3 suppresses the oxidative decomposition of LPSCl due to the enhanced chemical stability of Li2SnO3 with LPSCl. This reveals that the oxidative decomposition of LPSCl on the nanostructured LiCoO2 surface contributes more significantly to void formation than the volume change of LiCoO2. These findings provide valuable insights into the degradation mechanisms of nanostructured cathode materials.
中文翻译:

Li6PS5Cl基全固态电池纳米结构LiCoO2的界面降解机制
Li 6 PS 5 Cl (LPSCl) 在循环过程中与氧化物正极材料的界面降解,特别是界面空隙的形成,导致电化学性能较差。这些空隙的形成是由两种不同的机制驱动的:氧化物正极材料在循环过程中的体积变化以及LPSCl由于氧化分解而发生的体积收缩。然而,每种途径对空隙形成的相对贡献仍然不明确,特别是对于纳米结构阴极材料。本研究强调了与 LiCoO 2 在充电和放电之间的体积变化相比,LPSCl 的氧化分解对纳米结构 LiCoO 2 表面在界面空隙形成中的主要影响。比较了裸 LiCoO 2 和 LiCoO 2 –Li 2 SnO 3 核壳纳米粒子的界面降解行为。两种类型的纳米颗粒由于其相似的粒径和可逆容量,在循环过程中表现出相当的 LiCoO 2 绝对体积变化,有效排除了 LiCoO 2 体积变化对空隙的影响形成。然而,与裸露的LiCoO 2 相比,LiCoO 2 –Li 2 SnO 3 显示出减少的界面空隙形成,从而改善了电化学性能。这是由于 LiCoO 2 –Li 2 SnO 3 由于 Li 2 与 LPSCl。 这表明纳米结构LiCoO 2 表面上LPSCl的氧化分解对空隙形成的贡献比LiCoO 2 的体积变化更显着。这些发现为纳米结构阴极材料的降解机制提供了有价值的见解。
更新日期:2024-05-08
中文翻译:

Li6PS5Cl基全固态电池纳米结构LiCoO2的界面降解机制
Li 6 PS 5 Cl (LPSCl) 在循环过程中与氧化物正极材料的界面降解,特别是界面空隙的形成,导致电化学性能较差。这些空隙的形成是由两种不同的机制驱动的:氧化物正极材料在循环过程中的体积变化以及LPSCl由于氧化分解而发生的体积收缩。然而,每种途径对空隙形成的相对贡献仍然不明确,特别是对于纳米结构阴极材料。本研究强调了与 LiCoO 2 在充电和放电之间的体积变化相比,LPSCl 的氧化分解对纳米结构 LiCoO 2 表面在界面空隙形成中的主要影响。比较了裸 LiCoO 2 和 LiCoO 2 –Li 2 SnO 3 核壳纳米粒子的界面降解行为。两种类型的纳米颗粒由于其相似的粒径和可逆容量,在循环过程中表现出相当的 LiCoO 2 绝对体积变化,有效排除了 LiCoO 2 体积变化对空隙的影响形成。然而,与裸露的LiCoO 2 相比,LiCoO 2 –Li 2 SnO 3 显示出减少的界面空隙形成,从而改善了电化学性能。这是由于 LiCoO 2 –Li 2 SnO 3 由于 Li 2 与 LPSCl。 这表明纳米结构LiCoO 2 表面上LPSCl的氧化分解对空隙形成的贡献比LiCoO 2 的体积变化更显着。这些发现为纳米结构阴极材料的降解机制提供了有价值的见解。
































 京公网安备 11010802027423号
京公网安备 11010802027423号