当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
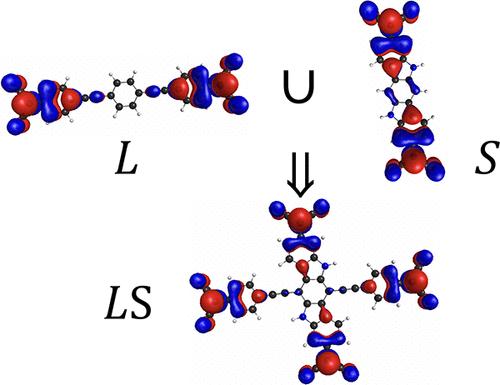
Pathway to Polyradicals: A Planar and Fully π-Conjugated Organic Tetraradical(oid)
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.jpclett.4c00686 Sergi Betkhoshvili 1 , Ibério de P. R. Moreira 2 , Jordi Poater 1, 3 , Josep Maria Bofill 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.jpclett.4c00686 Sergi Betkhoshvili 1 , Ibério de P. R. Moreira 2 , Jordi Poater 1, 3 , Josep Maria Bofill 1
Affiliation
|
In this work, we provide a general strategy to stabilize the ground state of polyradical(oid)s and make higher spin states thermally accessible. As a proof of concept, we propose to merge two planar fully π-conjugated diradical(oid)s to obtain a planar and cross-conjugated tetraradical(oid). Using multireference quantum chemistry methods, we show that the designed tetraradical(oid) is stabilized by aromaticity and delozalization in the π-system and has six thermally accessible spin states within 1.72 kcal/mol. Analysis of the electronic structure of these six states of the tetraradical(oid) shows that its frontier π-system consists of two weakly interacting subsystems: aromatic cycles and four unpaired electrons. Conjugation between unpaired electrons, which favors closed-shell structures, is mitigated by delocalization and the aromaticity of the bridging groups, leading to the synergistic cross-coupling between two diradical(oid) subunits to stabilize the tetraradical(oid) electronic structure.
中文翻译:

通向多自由基的途径:平面且完全 π 共轭的有机四自由基(oid)
在这项工作中,我们提供了一种总体策略来稳定多自由基的基态并使更高的自旋态可热接近。作为概念证明,我们建议合并两个平面完全 π 共轭二基(oid)以获得平面交叉共轭四基(oid)。使用多参考量子化学方法,我们表明所设计的四自由基(oid)通过 π 系统中的芳香性和解氮化而稳定,并且在 1.72 kcal/mol 范围内具有六种热可接近的自旋态。对四自由基这六种态的电子结构的分析表明,其前沿π系统由两个弱相互作用的子系统组成:芳香环和四个不成对电子。不成对电子之间的共轭有利于闭壳结构,通过离域和桥接基团的芳香性来减轻,导致两个二自由基(oid)亚基之间的协同交叉偶联,以稳定四自由基(oid)电子结构。
更新日期:2024-05-08
中文翻译:

通向多自由基的途径:平面且完全 π 共轭的有机四自由基(oid)
在这项工作中,我们提供了一种总体策略来稳定多自由基的基态并使更高的自旋态可热接近。作为概念证明,我们建议合并两个平面完全 π 共轭二基(oid)以获得平面交叉共轭四基(oid)。使用多参考量子化学方法,我们表明所设计的四自由基(oid)通过 π 系统中的芳香性和解氮化而稳定,并且在 1.72 kcal/mol 范围内具有六种热可接近的自旋态。对四自由基这六种态的电子结构的分析表明,其前沿π系统由两个弱相互作用的子系统组成:芳香环和四个不成对电子。不成对电子之间的共轭有利于闭壳结构,通过离域和桥接基团的芳香性来减轻,导致两个二自由基(oid)亚基之间的协同交叉偶联,以稳定四自由基(oid)电子结构。
































 京公网安备 11010802027423号
京公网安备 11010802027423号