当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct dehydroxy(sulfhydryl)xanthylation of alcohols and thiols
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2024-05-13 , DOI: 10.1039/d4qo00608a Dingjian Shan 1 , Menglin Jiang 1 , Fangcan Liang 1 , Yu Zhong 1 , Dianhu Zhu 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2024-05-13 , DOI: 10.1039/d4qo00608a Dingjian Shan 1 , Menglin Jiang 1 , Fangcan Liang 1 , Yu Zhong 1 , Dianhu Zhu 1
Affiliation
|
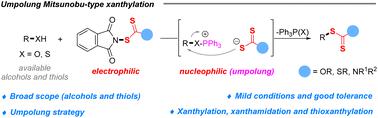
Alkyl xanthates are broadly applied as synthetic intermediates for expedient access to a range of functional group derivatives in synthetic chemistry. Dehydroxyxanthylations of naturally rich alcohols are a promising approach but have rarely been explored mainly due to their challenging activation mode. Herein, we report the direct dehydroxyxanthylation of a wide range of primary and secondary alcohols via easily accessible and shelf-stable electrophilic N-xanthyl phthalimides under mild conditions, with superb functional group compatibility toward cyano and nitro groups, unprotected active groups (–NH2 and –OH), and double and triple bonds. The present organic halide-free protocol might provide a new and green avenue toward the synthesis of alkyl xanthates in an umpolung Mitsunobu-type manner in organic synthesis. Additionally, aliphatic thiols successfully undergo desulfhydrylxanthylation to produce the corresponding sp3C-xanthates. Notably, the xanthamide and thioxanthate groups could also be transformed to the desired alkyl substrates via this umpolung strategy. The broad substrate scope, excellent functional group compatibility and late-stage functionalization of bioactive molecules makes this protocol very attractive as a novel strategy for the rapid incorporation of SC(S)R (R = OEt, Oalkyl, NEt2 and SEt) onto the alkyl chains of complex systems.
中文翻译:

醇和硫醇的直接脱羟基(巯基)黄乙酰化
烷基黄原酸盐广泛用作合成中间体,可在合成化学中方便地获得一系列官能团衍生物。天然丰富的醇的脱羟基黄基化是一种有前途的方法,但很少被探索,主要是由于其具有挑战性的激活模式。在此,我们报道了在温和条件下通过易于获得且储存稳定的亲电子 N-黄基邻苯二甲酰亚胺对各种伯醇和仲醇进行直接脱羟基黄酰化,对氰基和硝基、未保护的活性基团 (–NH < b0> 和 –OH),以及双键和三键。目前的无有机卤化物方案可能为有机合成中以umpolung Mitsunobu型方式合成烷基黄原酸盐提供一条新的绿色途径。此外,脂肪族硫醇成功地进行脱硫基黄原酸化,生成相应的 sp 3 C-黄原酸酯。值得注意的是,黄酰胺和硫代黄原酸基团也可以通过这种反极性策略转化为所需的烷基底物。广泛的底物范围、出色的官能团相容性和生物活性分子的后期功能化使该方案作为快速掺入 SC(S)R (R = OEt、O烷基、NEt 2
更新日期:2024-05-13
中文翻译:

醇和硫醇的直接脱羟基(巯基)黄乙酰化
烷基黄原酸盐广泛用作合成中间体,可在合成化学中方便地获得一系列官能团衍生物。天然丰富的醇的脱羟基黄基化是一种有前途的方法,但很少被探索,主要是由于其具有挑战性的激活模式。在此,我们报道了在温和条件下通过易于获得且储存稳定的亲电子 N-黄基邻苯二甲酰亚胺对各种伯醇和仲醇进行直接脱羟基黄酰化,对氰基和硝基、未保护的活性基团 (–NH < b0> 和 –OH),以及双键和三键。目前的无有机卤化物方案可能为有机合成中以umpolung Mitsunobu型方式合成烷基黄原酸盐提供一条新的绿色途径。此外,脂肪族硫醇成功地进行脱硫基黄原酸化,生成相应的 sp 3 C-黄原酸酯。值得注意的是,黄酰胺和硫代黄原酸基团也可以通过这种反极性策略转化为所需的烷基底物。广泛的底物范围、出色的官能团相容性和生物活性分子的后期功能化使该方案作为快速掺入 SC(S)R (R = OEt、O烷基、NEt 2
































 京公网安备 11010802027423号
京公网安备 11010802027423号