当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
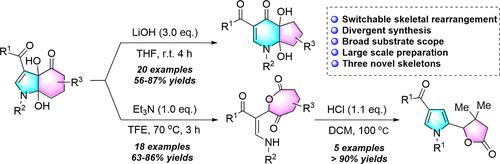
Switchable Skeletal Rearrangement of Hexahydro-4H-indol-4-ones: Divergent Synthesis of Dihydroxy-4H-cyclopenta[b]pyridin-4-ones and 8-Alkenyl Oxepane-2,6-diones
Organic Letters ( IF 5.2 ) Pub Date : 2024-05-14 , DOI: 10.1021/acs.orglett.4c00908 Zhilai Zhang 1 , Haifeng Sun 1 , Mingshuai Zhang 1 , Siyu Song 1 , Menglin Peng 1 , Weifeng Dai 1 , Yongchao Wang 2 , Fuchao Yu 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-05-14 , DOI: 10.1021/acs.orglett.4c00908 Zhilai Zhang 1 , Haifeng Sun 1 , Mingshuai Zhang 1 , Siyu Song 1 , Menglin Peng 1 , Weifeng Dai 1 , Yongchao Wang 2 , Fuchao Yu 1
Affiliation
|
An unprecedented base-controlled selective skeletal rearrangement reaction of hexahydro-4H-indol-4-ones has been developed. In this protocol, highly functionalized dihydroxy-4H-cyclopenta[b]pyridin-4-ones and 8-alkenyl oxepane-2,6-diones were prepared with a broad substrate scope and high chemoselectivity in moderate to excellent yields selectively by modulating LiOH and Et3N. In addition, the newly formed 8-alkenyl oxepane-2,6-dione scaffolds could be easily further derivatized to 5-(pyrrol-2-yl)dihydrofuran-2(3H)-ones through a rare intramolecular rearrangement reaction.
中文翻译:

六氢-4H-吲哚-4-酮的可切换骨架重排:二羟基-4H-环戊[b]吡啶-4-酮和8-烯基氧杂环庚烷-2,6-二酮的不同合成
开发了一种前所未有的六氢-4H-吲哚-4-酮的碱基控制选择性骨架重排反应。在该方案中,通过调节 LiOH 和Et 3 N。此外,新形成的8-烯基氧杂环庚烷-2,6-二酮支架可以很容易地进一步衍生化为5-(吡咯-2-基)二氢呋喃-2(3H)-酮通过罕见的分子内重排反应。
更新日期:2024-05-14
中文翻译:

六氢-4H-吲哚-4-酮的可切换骨架重排:二羟基-4H-环戊[b]吡啶-4-酮和8-烯基氧杂环庚烷-2,6-二酮的不同合成
开发了一种前所未有的六氢-4H-吲哚-4-酮的碱基控制选择性骨架重排反应。在该方案中,通过调节 LiOH 和Et 3 N。此外,新形成的8-烯基氧杂环庚烷-2,6-二酮支架可以很容易地进一步衍生化为5-(吡咯-2-基)二氢呋喃-2(3H)-酮通过罕见的分子内重排反应。
































 京公网安备 11010802027423号
京公网安备 11010802027423号