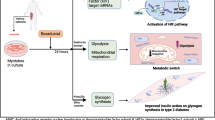
Abstract
Heart failure is a prevalent disease worldwide. While it is well accepted that heart failure involves changes in myocardial energetics, what alterations that occur in fatty acid oxidation and glucose oxidation in the failing heart remains controversial. The goal of the study are to define the energy metabolic profile in heart failure induced by obesity and hypertension in aged female mice, and to attempt to lessen the severity of heart failure by stimulating myocardial glucose oxidation. 13-Month-old C57BL/6 female mice were subjected to 10 weeks of a 60% high-fat diet (HFD) with 0.5 g/L of Nω-nitro-l-arginine methyl ester (L-NAME) administered via drinking water to induce obesity and hypertension. Isolated working hearts were perfused with radiolabeled energy substrates to directly measure rates of myocardial glucose oxidation and fatty acid oxidation. Additionally, a series of mice subjected to the obesity and hypertension protocol were treated with a pyruvate dehydrogenase kinase inhibitor (PDKi) to stimulate cardiac glucose oxidation. Aged female mice subjected to the obesity and hypertension protocol had increased body weight, glucose intolerance, elevated blood pressure, cardiac hypertrophy, systolic dysfunction, and decreased survival. While fatty acid oxidation rates were not altered in the failing hearts, insulin-stimulated glucose oxidation rates were markedly impaired. PDKi treatment increased cardiac glucose oxidation in heart failure mice, which was accompanied with improved systolic function and decreased cardiac hypertrophy. The primary energy metabolic change in heart failure induced by obesity and hypertension in aged female mice is a dramatic decrease in glucose oxidation. Stimulating glucose oxidation can lessen the severity of heart failure and exert overall functional benefits.







Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author (Dr Gary D. Lopaschuk).
Abbreviations
- HF:
-
Heart failure
- HFD:
-
High-fat diet
- L-NAME:
-
Nω-Nitro-l-arginine methyl ester
- PDKi:
-
Pyruvate dehydrogenase kinase inhibitor
- ATP:
-
Adenosine 5'-triphosphate
- IP:
-
Intraperitoneal
- %EF:
-
Ejection fraction
- %FS:
-
Fractional shortening
- %GLS:
-
Global longitudinal strain
- PV:
-
Pressure–volume
- MABP:
-
Mean arterial blood pressure
- SBP:
-
Systolic blood pressure
- LVID; s:
-
Left ventricular internal diameter
- CSA:
-
Cross-sectional area
- DCA:
-
Dichloroacetate
- PDH:
-
Pyruvate dehydrogenase
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- FOXO1:
-
Forkhead box protein O1
- MCD:
-
Malonyl-CoA decarboxylase
- BDH1:
-
ß-Hydroxybutyrate dehydrogenase 1
References
Allard M, Schonekess B, Henning S, English D, Lopaschuk GD (1994) Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 267:H742–H750. https://doi.org/10.1152/ajpheart.1994.267.2.H742
Badolia R, Ramadurai DK, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M (2020) The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circulation 142:259–274. https://doi.org/10.1161/CIRCULATIONAHA.119.044452
Barger PM, Kelly DP (1999) Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci 318:36–42. https://doi.org/10.1097/00000441-199907000-00006
Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K (1994) Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol 23:1617–1624. https://doi.org/10.1016/0735-1097(94)90665-3
Bøgh N, Hansen ES, Omann C, Lindhardt J, Nielsen PM, Stephenson RS, Laustsen C, Hjortdal VE, Agger P (2020) Increasing carbohydrate oxidation improves contractile reserves and prevents hypertrophy in porcine right heart failure. Sci Rep 10:8158. https://doi.org/10.1038/s41598-020-65098-7
Brownsey RW, Boone AN, Allard MF (1997) Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc Res 34:3–24. https://doi.org/10.1016/s0008-6363(97)00051-5
Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED (2005) Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146:5341–5349. https://doi.org/10.1210/en.2005-0938
Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA (2016) Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res 119:1177–1182. https://doi.org/10.1161/CIRCRESAHA.116.309769
Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ (2002) Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40:271–277. https://doi.org/10.1016/s0735-1097(02)01967-8
Dyck JR, Cheng J-F, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G (2004) Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res 94:e78–e84
Emelyanova L, Boukatina A, Myers C, Oyarzo J, Lustgarten J, Shi Y, Jahangir A (2019) High calories but not fat content of lard-based diet contribute to impaired mitochondrial oxidative phosphorylation in C57BL/6J mice heart. PLoS ONE 14:e0217045. https://doi.org/10.1371/journal.pone.0217045
Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, Crespo-Leiro MG, Vieites MG, Bianchi K, Morales V (2020) Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab 2:1223–1231. https://doi.org/10.1038/s42255-020-00276-5
Gopal K, Al Batran R, Altamimi TR, Greenwell AA, Saed CT, Dakhili SAT, Dimaano MTE, Zhang Y, Eaton F, Sutendra G (2021) FoxO1 inhibition alleviates type 2 diabetes-related diastolic dysfunction by increasing myocardial pyruvate dehydrogenase activity. Cell Rep. https://doi.org/10.1016/j.celrep.2021.108935
Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K (2006) Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc Res 72:430–437. https://doi.org/10.1016/j.cardiores.2006.08.020
Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JR, Ussher JR, Muoio DM (2019) Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 115:1606–1616. https://doi.org/10.1093/cvr/cvz045
Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S (2007) A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci 113:205–212
Hyyti OM, Ledee D, Ning X-H, Ge M, Portman MA (2010) Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am J Physiol Heart Circ Physiol 299:H868–H875. https://doi.org/10.1152/ajpheart.00931.2009
Karwi QG, Wagg CS, Altamimi TR, Uddin GM, Ho KL, Darwesh AM, Seubert JM, Lopaschuk GD (2020) Insulin directly stimulates mitochondrial glucose oxidation in the heart. Cardiovasc Diabetol 19:1–14. https://doi.org/10.1186/s12933-020-01177-3
Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ (2003) Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol 41:293–299. https://doi.org/10.1016/s0735-1097(02)02714-6
Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T (2010) Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3:420–430. https://doi.org/10.1161/CIRCHEARTFAILURE.109.888479
Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T (2010) Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3:420–430. https://doi.org/10.1161/CIRCHEARTFAILURE.109.888479
Larsen TS, Belke DD, Sas R, Giles WR, Severson DL, Lopaschuk GD, Tyberg JV (1999) The isolated working mouse heart: methodological considerations. Pflugers Arch 437:979–985. https://doi.org/10.1007/s004240050870
Lassers BW, Kaijser L, Carlson LA (1972) Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3 H-palmitate. Eur J Clin Invest 2:348–358. https://doi.org/10.1111/j.1365-2362.1972.tb00661.x
Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD (2002) High levels of fatty acids delay the recoveryof intracellular pH and cardiac efficiency inpost-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 39:718–725. https://doi.org/10.1016/s0735-1097(01)01803-4
Lopaschuk GD (2003) Pharmacologic rationale for trimetazidine in the treatment of ischemic heart disease. Am J Cardiovasc Drugs 3:21–26
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED (2021) Cardiac energy metabolism in heart failure. Circ Res 128:1487–1513. https://doi.org/10.1161/CIRCRESAHA.121.318241
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258. https://doi.org/10.1152/physrev.00015.2009
Lopaschuk GD, Wambolt R, Barr R (1993) An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 264:135–144
Martin C, Schulz R, Rose J, Heusch G (1998) Inorganic phosphate content and free energy change of ATP hydrolysis in regional short-term hibernating myocardium. Cardiovasc Res 39:318–326
Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY (2013) ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol 304:H1103-1113. https://doi.org/10.1152/ajpheart.00636.2012
Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY (2012) Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail 5:493–503
Neely JR, Morgan H (1974) Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 36:413–459. https://doi.org/10.1146/annurev.ph.36.030174.002213
Neubauer S (2007) The failing heart–an engine out of fuel. N Engl J Med 356:1140–1151. https://doi.org/10.1056/NEJMra063052
Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96:2190–2196. https://doi.org/10.1161/01.cir.96.7.2190
Pakiet A, Jakubiak A, Mierzejewska P, Zwara A, Liakh I, Sledzinski T, Mika A (2020) The effect of a high-fat diet on the fatty acid composition in the hearts of mice. Nutrients 12:824
Pecháňová O, Bernatova I, Pelouch V, Babal P (1999) L-NAME-induced protein remodeling and fibrosis. Physiol Res 48:353–362
Piao L, Sidhu VK, Fang Y-H, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD (2013) FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl) 91:333–346. https://doi.org/10.1007/s00109-012-0982-0
Randle P, Garland P, Hales C, Newsholme E (1963) The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 281:785–789. https://doi.org/10.1016/s0140-6736(63)91500-9
Rider OJ, Apps A, Miller JJ, Lau JY, Lewis AJ, Peterzan MA, Dodd MS, Lau AZ, Trumper C, Gallagher FA (2020) Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ Res 126:725–736. https://doi.org/10.1161/CIRCRESAHA.119.316260
Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV (2019) Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568:351–356. https://doi.org/10.1038/s41586-019-1100-z
Schmidt-Schweda S, Holubarsch C (2000) First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci 99:27–35
Schroeder MA, Lau AZ, Chen AP, Gu Y, Nagendran J, Barry J, Hu X, Dyck JR, Tyler DJ, Clarke K (2013) Hyperpolarized 13C magnetic resonance reveals early-and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail 15:130–140. https://doi.org/10.1093/eurjhf/hfs192
Shipp JC, Opie LH, Challoner D (1961) Fatty acid and glucose metabolism in the perfused heart. Nature 189:1018–1019. https://doi.org/10.1038/1891018a0
Stacpoole PW, Henderson GN, Yan Z, James MO (1998) Clinical pharmacology and toxicology of dichloroacetate. Environ Health Perspect 106:989–994. https://doi.org/10.1289/ehp.98106s4989
Stanley WC, Morgan EE, Huang H, McElfresh TA, Sterk JP, Okere IC, Chandler MP, Cheng J, Dyck JR, Lopaschuk GD (2005) Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol-Heart Circ Physiol 289:H2304–H2309
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129. https://doi.org/10.1152/physrev.00006.2004
Taniguchi M, Wilson C, Hunter CA, Pehowich DJ, Clanachan AS, Lopaschuk GD (2001) Dichloroacetate improves cardiac efficiency after ischemia independent of changes in mitochondrial proton leak. Am J Physiol Heart Circ Physiol 280:H1762–H1769. https://doi.org/10.1152/ajpheart.2001.280.4.H1762
Taylor LE, Ramirez LA, Musall JB, Sullivan JC (2019) Tipping the scales: Are females more at risk for obesity-and high-fat diet-induced hypertension and vascular dysfunction? Br J Pharmacol 176:4226–4242
Taylor M, Wallhaus TR, DeGrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK (2001) An evaluation of myocardial fatty acid and glucose uptake using PET with [18F] fluoro-6-thia-heptadecanoic acid and [18F] FDG in patients with congestive heart failure. J Nucl Med 42:55–62
Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, Lee DI, Yoo H, Kass DA, Szweda LI (2021) NAD+ repletion reverses heart failure with preserved ejection fraction. Circ Res 128:1629–1641. https://doi.org/10.1161/CIRCRESAHA.120.317046
Tran DH, May HI, Li Q, Luo X, Huang J, Zhang G, Niewold E, Wang X, Gillette TG, Deng Y (2020) Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat Commun 11:1771. https://doi.org/10.1038/s41467-020-15640-y
Ussher JR, Fillmore N, Keung W, Zhang L, Mori J, Sidhu VK, Fukushima A, Gopal K, Lopaschuk DG, Wagg CS (2016) Genetic and pharmacological inhibition of malonyl CoA decarboxylase does not exacerbate age-related insulin resistance in mice. Diabetes 65:1883–1891. https://doi.org/10.2337/db15-1145
Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD (2009) Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet–induced obese mice lacking malonyl CoA decarboxylase. Diabetes 58:1766–1775. https://doi.org/10.2337/db09-0011
Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS (2012) Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 94:359–369. https://doi.org/10.1093/cvr/cvs129
Wang S, Wang C, Turdi S, Richmond KL, Zhang Y, Ren J (2018) ALDH2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: role of CaM kinase II, histone H3K9 methyltransferase SUV39H, Sirt1, and PGC-1α deacetylation. Int J Obes (Lond) 42:1073–1087. https://doi.org/10.1038/s41366-018-0030-4
Wang Z, Li L, Zhao H, Peng S, Zuo Z (2015) Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism 64:917–925. https://doi.org/10.1016/j.metabol.2015.04.010
Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ (1988) Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiol 61:65–70. https://doi.org/10.1016/0002-9149(88)91306-9
Wisneski JA, Gertz EW, Neese RA, Mayr M (1987) Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest 79:359–366. https://doi.org/10.1172/JCI112820
Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY (2013) Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res 97:676–685. https://doi.org/10.1093/cvr/cvs424
Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD (2013) Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 6:1039–1048. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000228
Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD (2011) Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res 89:148–156
Zhang Y, Taufalele PV, Cochran JD, Robillard-Frayne I, Marx JM, Soto J, Rauckhorst AJ, Tayyari F, Pewa AD, Gray LR (2020) Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat Metab 2:1248–1264
Zhou Y-Q, Foster FS, Nieman BJ, Davidson L, Chen XJ, Henkelman RM (2004) Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics 18:232–244. https://doi.org/10.1152/physiolgenomics.00026.2004
Funding
Canadian Institutes of Health Research, 154302, Gary Lopaschuk.
Author information
Authors and Affiliations
Contributions
QS and GDL designed the study. JRBD, GYO, and GDL initiated and supervised the project. QS, CSW, BG, KW, AAO, HS, LZ, AV, BC, NW, and FW performed the experiments. QS, CSW, AAO, and HS analyzed the data. QS and GDL wrote the original draft of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GDL is a shareholder of Metabolic Modulators Research Ltd and has received grant support from Servier, Boehringer Ingelheim, Sanofi, and REMED Biopharmaceuticals. The other authors have no additional conflicts of interest relevant to this article to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Q., Wagg, C.S., Güven, B. et al. Stimulating cardiac glucose oxidation lessens the severity of heart failure in aged female mice. Basic Res Cardiol 119, 133–150 (2024). https://doi.org/10.1007/s00395-023-01020-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-023-01020-2