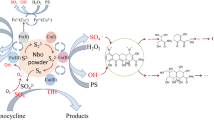
Abstract
This study investigated the use of heat-activated persulfate (HAP) as a chemical oxidation technique for removing tinidazole (TNZ) antibiotic from aqueous solutions. The impact of various operating parameters, including TNZ initial concentration (20 μM), persulfate (PS) initial dose (0.2–2 mM), solution pH (3–11), solution temperature (20–60 °C), and reaction time (10–120 min), was examined. The results indicated that sulfate radicals were the primary species responsible for TNZ degradation. Higher temperatures and PS concentrations improved the process, while higher pH values and TNZ initial concentrations slowed it down. Additionally, chloride and bicarbonate ions reduced reaction rates, with chloride ions having a more significant effect. Under optimal conditions (including [TNZ]0 = 20 μM, pH = 7, [PS]0 = 1 mM, temperature = 60 °C, and reaction time = 120 min), the removal efficiency achieved was 91.15%, with a mineralization rate of 85.8%. These results suggest that the process is relatively safe. The degradation of TNZ was best described by the pseudo-first-order model compared to other models. Additionally, the process was found to be exothermic and spontaneous, with a negative Gibbs free energy change indicating that it is thermodynamically feasible. The study found HAP to be an effective and cost-efficient technique for removing TNZ antibiotic due to its ease of operation and the absence of the need for additional chemicals or waste handling. Based on these findings, HAP can be considered an advanced oxidation technique for treating antibiotic-contaminated water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In today’s world, the use of drugs to maintain public health and improve the quality of life of humans is essential, so large amounts of drugs are produced daily worldwide and used to prevent diseases of humans and animals (Grenni et al. 2018; Almasi et al. 2016; Bazrafshan et al. 2021; Moghaddam et al. 2023; Dargahi et al. 2023; Shokoohi et al. 2018). Unfortunately, a large percentage of administered drugs (30–90%) is excreted unaltered or as active metabolites in urine and feces. This leads to their presence in wastewater, which is then directed to wastewater treatment plants (WWTPs) (Grenni et al. 2018; Shokoohi et al. 2020). However, adequate removal of these substances cannot be achieved due to the WWTPs’ reliance on microbial activity for operation. As a result, the effluent from WWTPs is recognized as the major anthropogenic source for the discharge of antibiotic residues (McConnell et al. 2018). Indeed, the widespread occurrence of such contaminants poses a serious challenge due to their adverse effects on both human health and ecosystems (Foroughi et al. 2018).
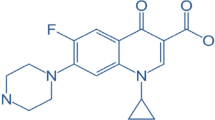
Nitroimidazoles are a class of antibiotics widely used to treat infections caused by anaerobic bacteria and protozoa such as Trichomonas vaginalis and Giardia lamblia. They are also used as growth promoters and for feeding improvement in poultry (Rivera-Utrilla et al. 2010a). Tinidazole (TNZ), the most well-known member of this antibiotic family, has high water solubility, raised toxicity, and low biodegradability. As with other antibiotics, TNZ cannot be removed by conventional biologically-based WWTPs (Velo-Gala et al. 2017a). Due to the reasons mentioned earlier, there is a growing need for effective elimination of these challenging compounds.
Several techniques such as (electro) chemical-based techniques, oxidation by ozone, gamma irradiation, photolysis, and adsorption have been tried until now (Zarei et al. 2013; Rivera-Utrilla et al. 2010b; Sánchez-Polo et al. 2009; Velo-Gala et al. 2017b; Li et al. 2022). Among these methods, advanced oxidation processes (AOPs) are considered the most promising and popular alternative according to research published in the Water Research journal. These methods rely on the generation of reactive oxidation agents, including hydroxyl radical (OH•) and sulfate (SO4•−) radicals, for the degradation of the compound (Foroughi et al. 2017). Among AOP-based processes, in situ chemical oxidation is considered an attractive option. Persulfate ion is one of the most effective oxidants utilized for remediation of organic pollutants. Its application has numerous advantages, including high oxidizing properties and water solubility, ease of storage and transportation, lower cost compared to other oxidants, safe generation of by-products, and proper persistence (Zarei et al. 2017).
In order to enhance the reaction rate of persulfate (PS) with reductants, it is preferable to decompose or stimulate it to sulfate radical (SO4•−) through an activation process. Common methods for achieving this include heat, transition metals, UV light, or base (Johnson et al. 2008; Ji et al. 2015a). SO4•− (E0 = 2.6) has superior oxidation ability compared to PS and can effectively decompose contaminants, even under neutral pH conditions. In addition, it can selectively oxidize target molecules with carbon–carbon double bonds and benzene rings more effectively than PS (Qi et al. 2018). Among various activation strategies, heat activation is particularly promising when integrated with in situ chemical oxidation (ISCO) (Zarei et al. 2017). In addition to producing SO4•−, heat can increase reaction rates, locally raise site temperature, and reduce cleanup time, which is especially important for large-scale applications. Moreover, thermally activated processes have fewer controversial issues related to chemical synthesis, handling, and waste generation than metal-catalyzed processes (Zhang et al. 2018; Qiu et al. 2022; Xu et al. 2023; Zou et al. 2022; Faria et al. 2019).
The objective of this study was to investigate the effect of heat-activated persulfate (HAP) on tinidazole (TNZ), a model antiprotozoal and antibiotic drug. TNZ was selected due to its appropriate characteristics, including low reactivity to ozone, high affinity for OH•, and a low adsorption rate on activated carbon, as well as its chemically complex structure. Furthermore, there are few reports on the capability of different treatment options for removing nitroimidazoles, which have recently been detected in environmental matrices such as aqueous media (9). The investigation focused on the impact of influential parameters such as TNZ initial concentration (20 μM), pH (3–11), PS initial dose (0.2–2), temperature (20–60 °C), and reaction time (10–120 min) on the process kinetics, isotherms, and thermodynamics. The mineralization of the process was also determined.
In summary, the study offers a holistic approach, combining the unique challenges posed by nitroimidazoles, the integration of heat activation with ISCO, and a thorough exploration of influential parameters, contributing valuable insights to the field of wastewater remediation.
Materials and methods
Chemicals and instruments
All the chemicals used in this study were of analytical pure grade. TNZ (purity > 99%, Merck, Germany) was acquired from Merck. Na2S2O8 (99%, Merck, Germany) was used as the persulfate agent. The samples’ pH was adjusted by adding either 0.1 N HCl or NaOH solution and measured with a pH meter (model WTW, Germany). Ethanol and tert-butyl alcohol were added to the system as radical scavengers. Sodium chloride (NaCl, purity ≥ 99.9%) and sodium carbonate (Na2S2O8) or sodium bicarbonate (NaHCO3, purity ≥ 99.9%) were used to investigate the effect of chloride and alkalinity, respectively. All solutions were prepared using deionized water. TNZ concentrations were determined using a DR 5000 spectrophotometer at the predetermined maximum wavelength (λ) of 310 nm, and total organic carbon (TOC) was detected using a TOC analyzer ANATOC™ SERIES II according to Standard Methods for the Examination of Water and Wastewater (Faria et al. 2019).
Experiments
The effect of HAP on TNZ removal was investigated by varying the parameters of TNZ initial concentration (20 μM), pH (3–11), PS initial dose (0.2–2 mM), temperature (20–60 °C), and time (10–120 min). The degradation experiments were conducted in batch mode using a one-factor-at-a-time approach. For each experimental run, 200 mL of a known concentration of TNZ solution was prepared in a 250-mL Erlenmeyer flask, adjusted to the desired pH, and contacted with an ambient amount of persulfate. The flasks were agitated in a temperature-controlled shaker incubator at a constant stirring rate of 100 rpm. After the reaction, approximately 4 mL of the solution was extracted, immediately quenched with 1 mL of ethanol to prevent further possible oxidation reactions, and analyzed for TNZ. The removal efficiency was calculated using the following equation:
where RTNZ is the TNZ removal efficiency (%), and TNZi and TNZf indicate the concentrations of TNZ (mg/L) at the initial and final time points, respectively.
To identify the radical species formed during the process, ethanol and tert-butyl alcohol were employed as radical scavengers in molar ratios of 125/1 (either TBA/PS or EtOH/PS 700/1) before and after addition of persulfate. The TNZ and PS working solutions were freshly prepared. All experiments were performed in triplicate, and mean values were reported (Table 1).
Results and discussion
Effect of temperature
Figure 1 shows the efficiency of HAP on TNZ removal at different temperatures. As shown, increasing the temperature from 20 to 60 °C significantly enhanced TNZ degradation from 6.8% to 81.5% within 120 min. Moreover, the TNZ degradation process was best described by a pseudo-first-order kinetic model with R2 = 0.95. The rate constants obtained using Eq. (2) are summarized in Table 2.
where kobs refers pseudo-first-order degradation rate constant (min−1) and d refers to TZN concentrations (mM) at time t. As shown in Table 2, kobs increased from 0.3 × 10−3 min−1 to 7.1 × 10−3min−1 once temperature raised to 60 °C from 20 °C. This can be contributed to the increasing of thermal energy and enhancement of PS degradation rate, which in turn leads to increase of SO4•− formation (Eq. 1) and other oxidants (Eqs. 3–6) for reaction with the target compound (Qi et al. 2018). These findings suggest that HAP may be an effective method for removing TNZ from certain environments or substances, and that higher temperatures can increase its efficiency.
In addition, to better explain the thermal dependency, the reaction rate constant (kobs) was calculated using Eqs. 7 and 8:
where A and Ea represent the pre-exponential parameter and the apparent activation energy, respectively; R refers to the universal gas constant (8.314 Jmol−1 K−1), and T represents absolute temperature. By fitting the experimental data to Eq. 8, the value of Ea was found to be 84 kJ/mol (Fig. 1b), which falls within the normal range of 60–250 kJ/mol (Zhang et al. 2018). Moreover, the data of ln k1 against 1/T fit well with the Arrhenius equation (R2 = 0.99).
The predominant radical(s) involved
To determine the primary type of reactive radical species responsible for TNZ degradation, we utilized two scavengers, EtOH and TBA, at a concentration of 0.08 mM. EtOH is well established as an effective quenching agent for both \({\text{SO}}_{{4}}^{ \cdot - }\) and \({\text{OH}}^{ \cdot }\) radicals, with its reaction rate toward the hydroxyl radical being 50 times greater than that of sulfate radical. Conversely, TBA is known to be a suitable scavenger for \({\text{OH}}^{ \cdot }\) due to its reaction rate, which is 1000 times higher when compared to sulfate radicals (Eqs. 9–12) (Wang et al. 2018a). The rate constants of these scavengers reacting with the radicals were determined as follows:
Figure 2 shows that the TNZ oxidation process was inhibited in the presence of both radical scavengers, mainly EtOH, as the corresponding values of the rate constants for the pseudo-first model in the presence of EtOH and TBA decreased by 82.94% and 65.88%, respectively. The results showed that both hydroxyl radicals (\({\text{OH}}^{ \cdot }\)) and sulfate radicals (\({\text{SO}}_{{4}}^{ \cdot - }\)) existed in the solution and contributed to the degradation of TNZ simultaneously. Despite the addition of radical scavengers, there is still some \({\text{SO}}_{{4}}^{ \cdot - }\) which can react with TNZ, as shown in Eqs. 9–12 and Fig. 2. Therefore, it seems that sulfate radicals are the predominant oxidants in TNZ decomposition, which is consistent with previous reports by Chen et al. (2018), Norzaee et al. (2018), and Ji et al. (2015b) on the oxidation of organic matter with HAP.
Effect of initial concentration of PS
To optimize the PS initial dose, it was introduced at different concentrations from 0.2 to 2 mM at pH = 7, Temp = 60 °C, and [TNZ]0 = 20 μM. The results illustrated in Fig. 3 clearly showed that the TNZ removal efficiency significantly increased with increasing PS initial dose. The elimination efficiency of TNZ increased to more than 98% from 40% during 120 min at initial PS doses of 2 mM and 0.2 mM, respectively, mainly due to the improvement of sulfate radical production. The linear relationship between persulfate concentration and kobs (Fig. 3) confirms that the reaction between the generated radicals (OH• and SO4•−) with the target antibiotic is predominant in comparison with other possible side reactions. The higher level of oxidant generation at higher doses of persulfate is in close agreement with reports by Ji et al. (2015b) and Chen et al. (2017).
Effect of initial pH
It is well known that the production of active free radicals is the original mechanism in AOP-based processes. Solution pH has a detrimental effect on both the type and concentration of radicals contributing to the degradation of the target pollutant (Wang et al. 2018b; Liu et al. 2018; Mohammadi et al. 2017; Bazrafshan et al. 2012a, 2012b; Nguyen et al. 2006). The effect of initial pH values (3–11) on the process, along with their corresponding rate constants for TNZ decomposition, is illustrated in Fig. 4. As shown, there is a high correlation between process performance and pH. Additionally, at all pH values, TNZ degradation followed a pseudo-first-order reaction. Furthermore, TNZ decomposition accelerated as the solution pH increased, and kobs decreased from 0.025 to 0.0079 with an increase in pH from 3 to 11.
This phenomenon can be attributed to the following reasons: (i) Due to its higher redox potential, persulfate can be catalyzed to form \({\text{SO}}_{4}^{ \cdot - }\) at acidic conditions, resulting in an enhancement of TNZ oxidation, as the highest efficiency was achieved at pH = 3. (ii) With increasing pH up to 7, both \({\text{OH}}^{.}\) and \({\text{SO}}_{4}^{ \cdot - }\) contribute to the oxidation, while at higher pH values, the contribution of \({\text{OH}}^{.}\) would be increased. Since the ORP of \({\text{OH}}^{.}\) and its presence in an aqueous solution is lower than that of \({\text{SO}}_{4}^{ \cdot - }\), a reduced efficiency would be expected at such pH values, as observed in this study. Therefore, the lowest efficiency and kobs were achieved at pH = 11 as well. (iii) Additionally, at alkaline conditions, hydroxide ions can act as \({\text{SO}}_{4}^{ \cdot - }\) scavengers and transform them into hydroxyl radicals (Eq. 4). While the latter is an unselective radical, it may also contribute to reactions 15 and 16 and decrease TNZ degradation rate (Norzaee et al. 2018; Chen et al. 2017). Therefore, it can be concluded that the relationship between kobs and efficiency with pH is mainly due to pH governing the type and concentrations of produced radicals.
Effect of coexisting ions
Natural waters contain various ions that may compete with the targeted species for adsorption sites or react with active oxidants in adsorption and AOP-based processes, respectively. Since this is an important issue, especially from a real-scale application perspective, such effects should be investigated (Nguyen et al. 2006). Therefore, the interference of competitive ions, such as chloride and bicarbonate, at different concentrations (0–10 mM), on TNZ degradation by HAP was assessed.
The effect of chloride ion
The existence of chloride ions can generally decrease the efficiency of AOP processes in which sulfate and hydroxide radicals are the main oxidation agents (Liu et al. 2018). In the present study, it was also observed that TNZ removal using HAP followed the pseudo-first-order model well (with R2 for all cases > 0.97), and kobs decreased with an increase in Cl concentration (Fig. 5). This can be due to the reaction of OH• and SO4•− with chloride radicals, which leads to the generation of different chloride oxidation species, as listed in Eqs. 17–21 (Ji et al. 2015a; Chen et al. 2017; Wang et al. 2018b), all of which have a lower ORP than the two original oxidants (i.e., OH• and SO4•).
The effect of bicarbonate
Bicarbonate (HCO3−) and carbonate (CO32−) are two of the most important indicators of alkalinity in water solutions that can easily convert to each other, while their chemical equilibrium strongly depends on pH (Ji et al. 2015a; Chen et al. 2017). Several studies have shown that HCO3− and CO32− ions can act as radical scavengers and reduce the performance of OH• and SO4•−. The final product of such reactions is carbonate radicals, which react with organic carbon 2–3 times slower than their parents. Therefore, it is expected that an increase in HCO3− concentration would result in a decrease in AOP process efficiency. Figure 5 shows that at HCO3− concentrations between zero and 10 mM, the reaction rate constant decreased from 0.019 to 0.004, respectively. Additionally, it is clear from Fig. 5 that the inhibition effect is more pronounced at higher concentrations of HCO3−, which may be due to the greater pH decrease induced by the higher concentration, which, in turn, decreases the oxidation efficiency as discussed before.
Mineralization
Achieving high removal efficiency cannot be interpreted as complete elimination of the target pollutant, and it may decompose into other organic by-products. Hence, the extent of mineralization in a process can determine how effectively the process removes the pollutant, without any concern for the production of by-products, whose dangers are sometimes equal to or even greater than those of the virgin pollutant. TOC concentrations, along with mineralization measurements, are presented in Fig. 6. As shown, during the 120 min reaction, approximately 91.15%, 85.8%, and 78.25% TNZ removal, mineralization, and TOC removal, respectively, can be achieved. Lynn et al. reported that after 120 min of contact time, 95% of the antibiotic ciprofloxacin was mineralized by UV-activated persulfate process (Lin and Wu 2014). Additionally, in the study by Norzaee et al., more than 68% of ciprofloxacin was transformed into its mineral form within 75 min (Norzaee et al. 2018).
Comparison with the other studies
Table 3 summarizes the literature reports on the removal of antibiotics by the HAP process. To make performance comparable, the listed studies have been normalized by the main effective operational parameters. As shown in Table 3, the results of the present study are in close agreement with those reported by similar studies. From a performance point of view, the findings are very close to the results reported by other studies. (Ji et al. 2015a; Arvaniti et al. 2022a, 2022b; Seid-mohammadi et al. 2020; Hasani et al. 2023). This study also highlights various aspects in comparison with other reported works. For example, TNZ abatement has been observed at a more natural pH (i.e., at pH 7) than in other studies where high efficiency only occurred at acidic or basic pH values (Chen et al. 2018, 2017; Norzaee et al. 2018; Ji et al. 2015b). Furthermore, TOC and COD abatement, as the main parameters for comparing degradation processes, are not reported in most of the listed studies. Therefore, more studies still need to be explored to overcome technical and practical barriers in scaling up the process for big- and real-scale applications.
Conclusion
A clean and novel method based on thermally activated persulfate was successfully applied to remove TNZ from aqueous solutions. The main findings of this work can be categorized as follows:
-
Persulfate initial concentration can significantly increase TNZ removal efficiency mainly due to enhanced radical generation.
-
Sulfate radicals were found to be the main oxidation agent in the degradation of TNZ using the HAP process.
-
The process was also highly dependent on pH and temperature, with efficiency increasing at low pH values and high temperatures.
-
An 85% TOC abatement at the optimum conditions, where TNZ removal was found to be 91.15%, indicates efficient mineralization of the antibiotic.
Overall, the results showed that the HAP process can be considered a promising method for addressing the challenges related to high levels of recently detected TNZ in environmental matrices. However, more investigation is needed to evaluate the process at low TNZ concentrations as observed in real matrices. The effect of other interferences such as natural organic matter and coexisting anions and cations should also be considered.
Data availability
The dataset and analyzed during the current study are available from the corresponding authors on realistic demand.
References
Almasi A, Dargahi A, Mohammadi M, Azizi A, Karami A, Baniamerian F, Saeidimoghadam Z (2016) Application of response surface methodology on cefixime removal from aqueous solution by ultrasonic/photooxidation. Int J PharmTechnol 8(3):16728–16736
Arvaniti OS, Ioannidi AΑ, Politi A, Miserli K, Konstantinou I, Mantzavinos D, Frontistis Z (2022a) Dexamethasone degradation in aqueous medium by a thermally activated persulfate system: kinetics and transformation products. J Water Process Eng 49:103134
Arvaniti OS, Ioannidi AA, Mantzavinos D, Frontistis Z (2022b) Heat-activated persulfate for the degradation of micropollutants in water: a comprehensive review and future perspectives. J Environ Manag 318:115568
Bazrafshan E, Mostafapour FK, Mahvi AH (2012a) Phenol removal from aqueous solutions using pistachio-nut shell ash as a low cost adsorbent. Fresen Environ Bull 21(10):2962–2968
Bazrafshan E, KordMostafapoor F, Soori MM, Mahvi AH (2012b) Application of combined chemical coagulation and electrocoagulation process to carwash wastewater treatment. Fresenius Environ Bull 21(9a):2694–2701
Bazrafshan E, Zarei AA, Mohammadi L, Barahuie F, Zafar M (2021) Efficient tetracycline removal from aqueous solutions using ionic liquid modified magnetic activated carbon (IL@mAC). J Environ Chem Eng. 9(6):106570
Chen Y, Deng P, Xie P, Shang R, Wang Z, Wang S (2017) Heat-activated persulfate oxidation of methyl-and ethyl-parabens: effect, kinetics, and mechanism. Chemosphere 168:1628–1636
Chen Y, Chen W, Lei L, He Y, Wang X, Liang Y (2018) Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Environ Sci Pollut Res 25(15):14904–14913
Dargahi A, Samarghandi MR, Shabanloo A et al (2023) Statistical modeling of phenolic compounds adsorption onto low-cost adsorbent prepared from aloe vera leaves wastes using CCD-RSM optimization: effect of parameters, isotherm, and kinetic studies. Biomass Conv Bioref 13:7859–7873
Faria PC, Órfão JJM, Pereira MFR (2019) Activated carbon based materials for the removal of pharmaceuticals from aqueous solutions: a review. J Environ Manag 231:355–368
Foroughi M, Arezoomand HRS, Rahmani AR, Asgari G, Nematollahi D, Yetilmezsoy K et al (2017) Electrodegradation of tetracycline using stainless steel net electrodes: Screening of main effective parameters and interactions by means of a two-level factorial design. Korean J Chem Eng 34(11):2999–3008
Foroughi M, Rahmani AR, Asgari G, Nematollahi D, Yetilmezsoy K, Samarghandi MR (2018) Optimization of a three-dimensional electrochemical system for tetracycline degradation using box-behnken design. Fresenius Environ Bull 27(3):1914–1922
Grenni P, Ancona V, Barra CA (2018) Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39
Hasani K, Hosseini S, Gholizadeh H et al (2023) Enhancing the efficiency of electrochemical, Fenton, and electro-Fenton processes using SS316 and SS316/β-PbO2 anodes to remove oxytetracycline antibiotic from aquatic environments. Biomass Conv Bioref 13:11813–11830. https://doi.org/10.1007/s13399-021-01967-z
Ji Y, Dong C, Kong D, Lu J, Zhou Q (2015a) Heat-activated persulfate oxidation of atrazine: implications for remediation of groundwater contaminated by herbicides. Chem Eng J 263:45–54
Ji Y, Fan Y, Liu K, Kong D, Lu J (2015b) Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res 87:1–9
Johnson RL, Tratnyek PG, Johnson RO (2008) Persulfate persistence under thermal activation conditions. Environ Sci Technol 42(24):9350–9356
Li G, Zhao H, Guo P, Liu D (2022) Effective removal of tinidazole by MIL-53 (Al)-NDC metal-organic framework from aqueous solution. J Solid State Chem 310:123066
Lin CC, Wu MS (2014) Degradation of ciprofloxacin by UV/S2O82− process in a large photoreactor. J Photochem Photobiol A Chem 285:1–6
Liu L, Lin S, Zhang W, Farooq U, Shen G, Hu S (2018) Kinetic and mechanistic investigations of the degradation of sulfachloropyridazine in heat-activated persulfate oxidation process. Chem Eng J 346:515–524
McConnell MM, Truelstrup Hansen L, Jamieson RC, Neudorf KD, Yost CK, Tong A (2018) Removal of antibiotic resistance genes in two tertiary level municipal wastewater treatment plants. Sci Total Environ 643:292–300
Moghaddam AA, Mohammadi L, Bazrafshan E, Batool M, Behnampour M, Baniasadi M et al (2023) Antibiotics sequestration using metal nanoparticles: An updated systematic review and meta-analysis. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2023.121448
Mohammadi L, Bazrafshan E, Noroozifar M, Ansari-Moghaddam A, Barahuie F, Balarak D (2017) Adsorptive removal of benzene and toluene from aqueous environments by cupric oxide nanoparticles: kinetics and isotherm studies. J Chem. https://doi.org/10.1155/2017/2069519
Nguyen TA, Scott K, Kannan N, Anderson MA (2006) Adsorption and advanced oxidation processes for water treatment: impact of natural organic matter. In: Owen DM, Nicks RW Jr (eds) Water quality engineering in natural systems. John Wiley & Sons Inc, New Jersey, pp 171–198
Norzaee S, Taghavi M, Djahed B, Mostafapour FK (2018) Degradation of penicillin G by heat activated persulfate in aqueous solution. J Environ Manage 215:316–323
Qi C, Liu X, Lin C, Zhang X, Ma J, Tan H et al (2018) Degradation of sulfamethoxazole by microwave-activated persulfate for water treatment: Efficiency, mechanism and toxicity. Water Res 132:96–106
Qiu R, Zhang P, Feng G, Ni X, Miao Z, Wei L, Sun H (2022) Enhanced thermal activation of persulfate by coupling hydrogen peroxide for efficient degradation of pyrene. Chemosphere 303:135057
Rivera-Utrilla J, Sanchez-Polo M, Prados-Joya G, Ferro-Garcia MA, Bautista-Toledo I (2010a) Removal of tinidazole from waters by using ozone and activated carbon in dynamic regime. J Hazard Mater 174(1):880–886
Rivera-Utrilla J, Sánchez-Polo M, Prados-Joya G, Ferro-García MA, Bautista-Toledo I (2010b) Removal of tinidazole from waters by using ozone and activated carbon in dynamic regime. J Hazard Mater 174(1–3):880–886
Sánchez-Polo M, López-Peñalver J, Prados-Joya G, Ferro-García MA, Rivera-Utrilla J (2009) Gamma irradiation of pharmaceutical compounds, nitroimidazoles, as a new alternative for water treatment. Water Res 43(16):4028–4036
Seid-mohammadi A, Ghorbanian Z, Asgari G, Dargahi A (2020) Photocatalytic degradation of metronidazole (MNZ) antibiotic in aqueous media using copper oxide nanoparticles activated by H2O2/UV process: Biodegradability and kinetic studies. Desalin Water Treat 193:369–380
Shokoohi R, Gillani RA, Mahmoudi MM, Dargahi A (2018) Investigation of the efficiency of heterogeneous Fenton-like process using modified magnetic nanoparticles with sodium alginate in removing Bisphenol A from aquatic environments: kinetic studies. Desalin Water Treat 101:185–192
Shokoohi R, Dargahi A, AzamiGilan R, ZolghadrNasab H, Zeynalzadeh D, MollaMahmoudi M (2020) Magnetic multi-walled carbon nanotube as effective adsorbent for ciprofloxacin (CIP) removal from aqueous solutions: isotherm and kinetics studies. Int J Chem React Eng 18(2):20190130. https://doi.org/10.1515/ijcre-2019-0130
Velo-Gala I, Piran-Montano JA, Rivera-Utrilla J, Sánchez-Polo M, Mota AJ (2017a) Advanced Oxidation Processes based on the use of UVC and simulated solar radiation to remove the antibiotic tinidazole from water. Chem Eng J 323:605–617
Velo-Gala I, Pirán-Montaño JA, Rivera-Utrilla J, Sánchez-Polo M, Mota AJ (2017b) Advanced oxidation processes based on the use of UVC and simulated solar radiation to remove the antibiotic tinidazole from water. Chem Eng J 323:605–617
Wang B, Gao Y, Su C, Ma J, Liu Y, Zhang G et al (2018a) Response surface optimization of activated carbon production from bamboo sawdust for tetracycline removal. Bioresour Technol 265:291–304
Wang Z, Shao Y, Gao N, Lu X, An N (2018b) Degradation kinetic of phthalate esters and the formation of brominated byproducts in heat-activated persulfate system. Chem Eng J 334:2349–2357
Xu Q, Zhou F, Yu Q, Xiao Y, Jiang X, Zhang W, Qiu R (2023) Aniline degradation and As (III) oxidation and immobilization by thermally activated persulfate. Chemosphere 338:139573
Zarei AA, Tavassoli P, Bazrafshan E (2017) Evaluation of UV/S2O8 process efficiency for removal of metronidazole (MNZ) from aqu eous solutions. Water Sci Technol 1:126–133
Zarei A, Biglari H, Mobini M, Dargahi A, Ebrahimzadeh G, Narooie MR, Mehrizi EA, Yari AR, Mohammadi MJ, Baneshi MM, Khosravi R (2018) Disinfecting poultry slaughterhouse wastewater using copper electrodes in the electrocoagulation process. Pol J Environ Stud 27(4):1907–1912
Zhang X, Chen J, Sun H, Chai X, Jia J (2018) Effective removal of sulfamethoxazole from aqueous solution by activated carbon derived from Enteromorpha prolifera. J Environ Sci (china) 68:20–27
Zou Z, Huang X, Guo X, Jia C, Li B, Zhao E, Wu J (2022) Efficient degradation of imidacloprid in soil by thermally activated persulfate process: performance, kinetics, and mechanisms. Ecotoxicol Environ Saf 241:113815
Acknowledgements
This project was financially supported by Torbat Heydariyeh University of Medical Sciences under grant number A-10-1350-4. Also, the project is in accordance with the ethical principles and the national norms and standards for conducting Medical Research in Iran (IR.THUMS.REC.1398.010).
Funding
The current study was financially supported by Torbat Heydariyeh University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All authors had equal contributions in writing, review, and final approval of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this work.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarei, A.A., Bazrafshan, E., Mosafer, J. et al. In situ chemical oxidation of tinidazole in aqueous media by heat-activated persulfate: kinetics, thermodynamic, and mineralization studies. Appl Water Sci 14, 71 (2024). https://doi.org/10.1007/s13201-024-02133-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02133-2