Abstract
This study aimed at monitoring and management of the surface water and potentially pathogenic microbes of Lake Tonga (Algeria) with respect to. It characterized the main bacterial diversity patterns of Lake Tonga and predicted from water physicochemical parameters and water quality index (WQI) the distribution of bacterial species and the main indicator groups of faecal water contamination. Water samples were taken monthly at three sampling sites of different water depths. Several physicochemical parameters were measured; of which some were included in computing WQI to characterize the water quality of the lake. Counting, isolation and bacterial identification methods were used to characterize the existing aerobic heterotrophic bacteria. The composition of the microbial community of the waterbody of Lake Tonga included an abundant culturable bacterial flora belonging to several bacterial families and whose specific richness varied between water depths of the sites sampled. Species richness of the bacteria identified phenotypically varied between 7 and 11 per sample. The site with shallow water was the richest in bacterial species, compared to moderate and deep waters. The redundancy analysis showed the main physicochemical drivers of the microbial community composition. Our findings showed that high WQI scores indicated the water quality deterioration which triggered the increase in total load of faecal indicator bacterial groups. This study identified in Lake Tonga an important culturable aerobic bacterial flora whose specific richness and distribution varied spatially following the effects of water physicochemical parameters. Lake Tonga needs an integrated management plan to mitigate human disturbances declining water quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In nature, water is rarely found in its pure state because water quality is commonly influenced by human wastes, various pollutants and the introduction of pathogenic organisms (Chenchouni et al. 2022). The contamination of water resources leads to the emergence of diseases which are considered among the leading causes of death worldwide (Boyd 2020). Many fish and wildlife diseases can also be transmitted through water (Leung et al. 2019). Wastewater discharged into surface water can influence its physicochemical parameters, and introduce a significant load of non-indigenous microorganisms to the microbial community (Kalinowska et al. 2021). Water resides longer in lakes and ponds than in streams and lotic systems. As a consequence, longer retention time promotes changes in water quality due to interactions between physical, chemical, and biological parameters (Boyd 2020; Loucif et al. 2020).
Bacterial communities are an integral part of freshwater ecosystems. Bacteria have a significant impact on water quality, and their diversity, species and distribution generally reflect wetland water quality (Cotner and Biddanda 2002; Zwart et al. 2002; Logue and Lindström 2008; Newton et al. 2011; Yu et al. 2019). These microorganisms are responsible for much of the breakdown of organic matter and recycling of nutrients (Tanentzap et al. 2019). Therefore, the richer a waterbody is in organic matter, the more favorable conditions it provides for microbial growth, leads to an increase in the number and diversity of microorganisms (Boyd 2020; Kalinowska et al. 2021).
As the distribution of microorganisms varies in time and space (Remold et al. 2015), the prediction and/or determination of their ecosystem functions requires understanding the structure and composition of microbial communities, and diversity in relation to biotic and abiotic environmental components (Liu et al. 2012; Yu et al. 2019). Therefore, a better understanding of the variations in the interactions of microorganisms and their environment and the susceptibility these microorganisms to abiotic and biotic stressors has important implications for identifying the types of the selective stress model they undergo as well as the relationship between population dynamics and microbial community structure (Remold et al. 2015; Kalinowska et al. 2021).
Aquatic ecosystems are known to harbor high biodiversity, and despite significant research progress in this field, we still know very little about microbial diversity in terrestrial aquatic systems. The diversity, structure and stability of microbial communities therefore remain poorly characterized in many aquatic ecosystems, both terrestrial and marine and oceanic (Wilburn et al. 2019). In this sense, Lake Tonga (Algeria) represents an interesting ecosystem to explore microbial diversity due to its complex hydrology (Benyacoub et al. 2011). So far, no studies on the isolation and characterization of bacteria and other microbial populations of this lake have been conducted. This study is the first to identify bacterial species and investigate their diversity within this wetland classified Ramsar site and UNESCO Heritage Biosphere Reserve (BirdLife International 2020).
Aquatic ecosystems are the major receptors for nutrients and organic matter (Belhouchet et al. 2024). Indeed, sewage discharge and use of the surrounding landscape lead to significant changes in aquatic ecosystems through nutrient influx (Qu et al. 2017) and other pollutants (Eckert et al. 2018). These changes can lead to modification in bacterial community structure (Wang et al. 2017) and bacterial resistance (Alexander et al. 2020).
It has also been shown that ecosystems with high bacterial diversity are more resistant to invasion by new species (Dillon et al. 2005; van Elsas et al. 2012), and several studies have shown that bacterial cultures in well-mixed liquid lead to the collapse of bacterial communities. This is because, under these conditions, the strongest competitors take over (Kerr et al. 2002; Kim et al. 2008). The survival and abundance of bacterial species in a natural environment depend on environmental conditions. These conditions strongly influence the strength of bacterial selection, but the behavior of these microorganisms resulting from selection can in turn modify environmental conditions, thus promoting complex feedback loops (Mitri and Foster 2013; D'Souza et al. 2018). Many environments host diverse and dense microbial communities, and to understand microbes and their response to disturbances, it is essential to unravel the social interactions between strains and species of these microorganisms (Sachs and Hollowell 2012; Mitri and Foster 2013). Bacteria are in constant competition for a multitude of resources such as favorable living spaces and minerals. Moreover, due to their metabolic activities, bacteria significantly influence the metabolism of other co-existing organisms and transform the environments in which they live (González et al. 2015). The most complex ecosystems are the least stable. The complexity of an ecosystem is positively influenced by the abundance of bacterial species, cooperation, the strength of interactions (correlated with the level of interaction), the number of species with which each species interacts and competition. (Fox 2002; Mitri and Foster 2013; Cardona et al. 2018; D'Souza et al. 2018).
This study aimed at monitoring and managing surface waters of a lentic lake with respect to potentially pathogenic microbes. It considered the factors and different environmental conditions that favor the occurrence of bacterial species in these aquatic systems and targeted to predict from the physicochemical parameters of water the emergence and proliferation of potentially dangerous microbes in surface waters using statistical modeling approaches. We expect that findings of this study will help to understand the risk and danger associated with pathogenic organisms in freshwater ecosystems. This study analyzed the spatial and temporal patterns of the structure of the culturable bacterial flora in Lake Tonga. It examined also spatial and temporal similarity of the bacterial community. We analyzed the spatiotemporal variations of its composition while investigating the variability patterns in species richness and occurrence frequency of bacterial species according to sampling sites and times. Due to their universal uses as microbiological indicators of water quality and their faecal and environmental character (Guemmaz et al. 2020), the study analyzed the spatiotemporal distribution of groups of bacteria indicative of faecal contamination within the reserve of the Tonga Lake. We also addressed the question of how the bacterial community of Lake Tonga depends on the water physicochemical variables while investigating the influence of these parameters on the presence of bacterial species and their diversity in this aquatic environment.
Materials and methods
Study area
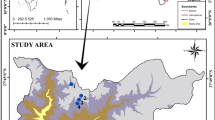
Lake Tonga (36° 51′ 51.1" N, 08° 30′ 10.0" E, elevation: 2 m a.s.l.), is located in the extreme north east of Algeria, at about 5 km west of the Tunisian border (Fig. 1). The site consists of a marshy basin and a shallow lake forming part of the complex of wetlands included in the National Park of El Kala, which is on the list of Ramsar sites since 1983, a Biosphere reserve, and an important bird area 'IBA' (Fishpool and Evans 2001; BirdLife International 2020). Covering an area of 2,400 ha, this ecosystem is considered important for the reproduction of many bird species in Algeria and one of the most important in the Mediterranean (BirdLife International 2020).
Location of Lake Tonga and sampling sites (S1–S3) in northeastern Algeria. The top right graph is the Gaussen and Bagnouls ombrothermal diagram of the region, where mean precipitation and air temperature are monthly averages for the period 1985–2016. The bottom map is the elevation map, i.e. presentation of the relief by altitudes, where the elevation is represented in a color scale and Lake Tonga is indicated by a black square
Lake Tonga hosts a large alder grove (Alnus glutinosa (L.) Gaertn.) along its northern shore, which represents a centerpiece of the site as this ensures an important functional role for the avifauna. Also, the floating vegetation rafts constitute one of the singularities of this lake (Benyacoub et al. 2011; Loucif et al. 2021). This lentic ecosystem is bounded to the north by a vast system of sand dunes, through which the lake connects to the Mediterranean Sea via an artificial channel, Oued Messida. According to climatic data provided by the El Kala station over a period of 22 years (1985–2016), the area is characterized by a humid climate with the De Martonne aridity index IDM = 24.7. The Emberger quotient of 112.7 positions the area in the subhumid bioclimatic stage with a warm winter variant. The ombrothermic diagram of Gaussen and Bagnouls delimits a dry season which extends from mid-May to mid-September (Loucif 2020).
Sampling sites of water
This study was conducted at Lake Tonga, whose northwestern shores of the lake are characterized by the presence of hamlets of rural dwellings in full extension with an increase in their wastewater discharges into the lake waterbody. We sampled water during the period from January to June 2018 at three sampling sites (S1, S2 and S3) chosen according to a depth gradient:
Site 1 (S1): located at the southern limit of the alder grove (Alnus glutinosa), it represents relatively shallow water (≈ 97 cm) located 540 m from the north shore and 1080 m from the west shore.
Site 2 (S2): represent a moderate depth of the lake, located towards the center at a depth of about 150 cm at 1020 m from the north shore and 1140 m from the west shore.
Site 3 (S3): located in a deeper area near the lake center towards the western shore. With a depth of 190 cm located 1600 m from the northern limit of the lake and 920 m from the western shore.
Water collection
For the physicochemical analysis, the water samples were collected in clean polypropylene bottles of 1.5 L capacity from a depth of 20 cm. The bacteriological analyzes were carried out on samples of water taken from sterile glass bottles with a capacity of 250 mL previously sterilized at 170 °C for 1 h. All water samples were hermetically sealed and correctly labelled. Once water collection was done, the vials were placed in situ in the dark in a cooler at low temperature (4 °C) and immediately returned to the laboratory for carrying out the analysis following standard methods and protocols (Rejsek 2002; Rodier et al. 2009).
Water physicochemical analyses
Several parameters were used to characterize the lake water: pH, electrical conductivity (EC), dissolved oxygen (DO), total suspended solids (TSS), turbidity, dry residues (Dry.res), five-day biological demand in oxygen (BOD5), total hardness, concentrations of calcium (Ca2+), magnesium (Mg2+), potassium (K+), chlorides (Cl−), sulphates (SO42−), phosphate (PO43−), ammonium (NH4+), nitrates (NO3−) and nitrites (NO2−). Some parameters, such as pH, DO (% saturation) and electrical conductivity of the water, were performed in situ using a WTW MultiLine® portable multi-parameter. The determination of suspended solids in the water was carried out at the laboratory level, by applying the filtration method on a glass fiber filter. It was expressed in mg/L (NF EN872 2005; Rodier et al. 2009). Determining the dry residues consisted of gradually evaporating a certain quantity of well-mixed water in a weighed capsule. Once all the water has evaporated, the dish is brought to an oven at 105 °C for 4 h and left to cool for 1/4 h in a desiccator. The dried residue was then weighed. The BOD5 measurement was carried out at the laboratory level with a WTW ''Oxitop System'' type device equipped with a cap having a pressure sensor which automatically follows the evolution of the biological oxygen demand during the oxidation of organic matter and keeps in memory the pressures at times 1, 2, 3, 4 and 5 days. BOD5 was expressed in mg O2/L. The determination of the total hardness was carried out by the titrimetric method with EDTA (ethylenediaminetetraacetic acid). Calcium, potassium, magnesium, ammonium, sulphates, phosphates, nitrates and nitrites concentrations were determined by ion spectrophotometry. The chloride concentration was determined using the flow injection analysis (FIA) method and photometric detection (NF EN ISO-15682, 2001); Rodier et al. 2009). The determination of calcium and potassium was carried out by spectrometry at a wavelength of 422.7 nm for calcium and 766.5 nm for potassium (Rodier et al. 2009). The sulphate ion assay method was based on precipitation in hydrochloric medium in the state of barium sulphate and performing the assay after spectrophotometer calibration; the spectrophotometer assay was performed at 420 nm (Rodier et al. 2009). Phosphate was measured at a wavelength of 430 nm (Rejsek 2002; Rodier et al. 2009). The dosage of ammonium ions was carried out at a wavelength of 630 nm. Magnesium was measured at a wavelength of 285.2 nm (Rodier et al. 2009). Nitrate and Nitrite ions were determined by spectrophotometry at 415 nm and 520–540 nm, respectively.
Bacteriological analyses
The methods used for the bacteriological examination of the water samples are divided into two main lines: counting methods and methods of isolation and identification. The first aimed at a quantitative estimate of the microbial flora of the analyzed waters, while the second aimed at characterizing the existing bacterial species. For each sample, total heterotrophic bacteria (THB), total coliforms (TC) and faecal coliforms (FC) were counted using the membrane filter method (Rodier et al. 2009). Colonies of faecal streptococci (FS) were counted by filtration on a cellulose membrane applied to a selective nutrient support consisted of Slanetz and Bartley medium. For bacterial identification, and in order to allow the growth of the majority of bacterial strains, seeding and re-isolations were carried out on non-selective media and selective media for each sample. For each sample, a mixture of different colonies was obtained which were studied individually. Microscopic observation and Gram staining have made it possible to characterize the different types of bacteria and to differentiate Gram-positive from Gram-negative bacteria. Then, the bacteria were isolated from the mixture and identified using the following culture media: nutrient agar, Hektoen enteric agar media, blood agar, Chapman medium, SS (Salmonella-Shigella) agar, Mac Conkey medium, and BCP (Bromo-Cresol Purple) medium. For the identification of the bacterial species, classical phenotypic and biochemical methods were used including oxidase test, study in TSI media, and catalase test. Miniaturized strips of API systems (API® 20 E, API® 20 Staph, API® 20 Strep) were used. After incubation of the API strips, reactions were noted as ( +) or (–), and we identified the bacterial species by referring to the reading table.
Water quality index (WQI)
The Water Quality Index (WQI) reduces water parameters measured to single digits to assess the overall water quality at a given location or date. A weight wi was assigned to each of the physicochemical parameters according to its importance in the overall quality of water and the possible of its effects on plants and health. The minimum weight 1 was assigned to parameters considered non-dangerous, while parameters that had the greatest effects on water quality had the highest weight of 5 (Bouderbala 2017). The relative weight (Wi) of each parameter was calculated using (Eq. 1).
with n is the number of physicochemical parameters.
In the current study, WQI was calculated using the drinking water quality standards recommended by the World Health Organization (WHO 2017). The results are presented in (Table 1).
In each water sample, the calculation of the qi rating scale for each parameter was obtained by dividing the concentration or observed value (Ci) by its respective standards (Si) defined according to WHO guidelines (WHO 2017). The results were multiplied by 100 (Eq. 2).
Before calculating WQI, the water quality sub-index (SI) is determined for each physicochemical parameter using (Eq. 3), then WQI was obtained by summing SI of n parameters (Eq. 4).
Depending on the WQI value, water quality can be classified into five categories (Li et al. 2014): excellent water quality (WQI < 25), good water quality (25 < WQI < 50), moderate or marginal water quality (50 < WQI < 100), poor water quality (100 < WQI < 150), and extremely poor water quality (WQI > 150).
Statistical analysis
Statistical analyses were carried out using the statistical software R version 4.2.1 (R Core Team 2022). The composition of bacterial species per sampling sites and months was visualized with a heatmap and cord diagram which included frequencies of species occurrences that were computed as the ratio between the number of samples where a given species occurred and the total number of samples. In the same vein, and using quantitative data (number of colony-forming units), the distribution of bacterial groups (THB, TC, FC and FS) was analyzed for sampling sites and months. Species richness (total number of species identified) was determined for each water sample, then the data were expressed using mean and standard deviation (SD) for sites and months. The variation in bacterial species richness and WQI values between the sampled sites and months was tested using two-way ANOVA. Using the package “venn” version 1.11 in R, the spatial and temporal similarities and overlap of species composition was explored between sites and between months within each sampled site. Using the “vegan” package in R (Oksanen et al. 2022), redundancy analysis (RDA) was employed to explore gradients of relationships linking the bacteria occurrences and water physicochemical parameters measured. The RDA triplot was designed using a correlative scaling method. Using RDA summary scores of the first five RDA axes, Pearson correlation tests were performed to examine the relationships between water parameter values observed during each month and sampling site and RDA site scores (weighted sums of response variable scores). For these correlations, statistical significance was considered at two thresholds: p = 0.05 and p = 0.10. The effect of WQI on the variation in bacterial species richness was tested using generalized linear models (GLM) with Gaussian distribution and identity link for the entire study period, and for the cold rainy season (mean minimum temperature = 9.2 °C, precipitation = 502 mm) as well as for the hot dry season (mean maximum temperature = 23.4 °C, precipitation = 98 mm). Same for the effects of WQI on bacterial loads of contamination-indicators where GLMs were used to test the variation of THB, TC, FC and FS as function of WQI for the entire study period, the cold rainy season and for the hot dry season.
Results
Bacterial species composition
The phenotypic identification of the bacterial strains isolated from the waterbody of Lake Tonga revealed 22 species belonging to 16 genera and five families. The Enterobacteriaceae family largely dominated with 59.1% of the species; it is followed by the Pseudomonadaceae family with 18.2%, Staphylococcaceae with 13.7% species, Burkholderiaceae and Enterococcaceae with 4.5% each. Pantoea agglomerans was identified at S1 and S2. The presence of Escherichia coli was reported throughout the study period in all samples (Fig. 2). Edwardsiella tarda was identified only once at S2 in May. Enterobacter cloacae was reported at all three sites (S1, S2 and S3); its presence was reported in two out of three samples during June and in a single sample for January, February, March and April. Enterococcus faecalis was more frequent at S3. The presence of Pectobacterium carotovorum was recorded at S1 and S3 with a frequency of occurrence of 16.7% and 33.3%, respectively. Cupriavidus necator was reported at S2 and S3. Klebseilla oxytoca was present throughout the study period with a greater abundance during May. Klebseilla pneumonia (Enterobacteriaceae) was more abundant in April and March. Morganella morganii was isolated from S1 and S2 and was identified from samples collected during the period January-April. Plesiomonas shigelloides was identified at S1 and S2.
Heatmap (upper plot) and cord diagram (bottom plot) displaying respectively the distribution of occurrence frequencies (in %) and abundances of bacterial species for sampling months and sites and at the waterbody of Lake Toga in extreme northeastern Algeria. Bacterial species binomial names are abbreviated using the first letter of the genus and four first letters of the species
Proteus mirabilis was isolated throughout the study period. Proteus vulgaris was identified at three sites with a high occurrence frequency in S1. Providencia rettgeri was identified at all sampling sites. Pseudomonas aeruginosa was isolated from the three sites with an occurrence frequency of 83.3% at S1. Pseudomonas fluorescens, was present in the three sites with a frequency of presence 66.7% recorded at each of the two sites S1 and S3. Pseudomonas putida was identified at all sampling sites throughout the study period; with S1 had more abundance of this germ with a frequency of presence of 100%. Pseudomonas syringae was reported only once at S1 during April, whereas Serratia marcescens was identified only once during February at S2. Staphylococcus aureus was encountered at all sites throughout the study period, the presence of this germ was more marked in sites S2 and S3 (83.3%). Staphylococcus epidermidis was isolated from all sites, the presence of this species was more marked in S1, whereas Staphylococcus intermedius was identified only at S3 during June (Fig. 2).
Spatiotemporal distribution of bacterial groups
For the total load of groups of bacteria indicators of faecal contamination, the first site (water depth = 97 cm), located north of Lake Tonga at the southern limit of the alder grove, contained a proportion of 36.8% of THB, 33.1% of TC, 26.4% of FC, and 3.7% of FS. The second site (water depth = 150 cm), located towards the center of the lake, harbored a proportion of 39.4% of THB, followed by proportions of TC and FC with 29.9% and 26.2%, respectively; whereas FS represented only 4.5% of the total load of groups of bacteria indicators of faecal contamination. The third site, located in a deeper zone (depth = 190 cm), was composed of 43% THB, 28.6% TC, 22.8% FC and 5.5% FS. THB and TC presented low loads in January, and a maximum load in May. The counts of CF showed a minimum in February and a maximum in May. For FS, the minimum was recorded in January and the maximum in May and June (Fig. 3).
Spatiotemporal variation of bacterial species richness
The number of bacterial species varied between 7 and 11 species per sample with an average of 8.9 ± 1.3 species (mean ± SD). The first site was the richest in bacterial species with an average of 10.2 ± 0.8 species compared to the other two sites which contained 8.8 ± 0.8 and 7.8 ± 1.0 species, respectively (Fig. 4). The variation in bacterial species richness between the sampled sites was significant (ANOVA: F(2,10) = 13.2, p = 0.002). The maximum number of species was recorded in April with 9.7 ± 0.6 species, while the minimum (8.3 ± 1.5 species) was observed in January and March with. However, no significant variation was observed for the variation in species richness between months (ANOVA: F(5,10) = 1.4, p = 0.312).
Boxplots showing distribution of species richness of water bacterial at sampling sites and months in Lake Tonga. Letters associated to means (solid white circles) are results of the honestly significant difference (HSD) of Tukey's range test. Means with different letters are significantly different (p < 0.05)
Spatial and temporal similarities of species composition
According to the Venn diagram, 13 bacterial species were shared between the three sites: Escherichia coli, Enterobacter cloacae, Enterococcus faecalis, Klebseilla oxytoca, Klebseilla pneumoniae, Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas putida, Staphylococcus aureus, and Staphylococcus epidermidis. Three species (Pantoea agglomerans, Morganella morganii, Plesiomonas shigelloides) were common species exclusively between S1–S2. Pectobacterium carotovorum was a common species exclusively between S1–S3, while Cupriavidus necator was exclusively a common species between S2–S3. A single bacterial species, Pseudomonas syringae, was exclusive to S1, two species: (Serratia marcesans and Edwardsiella tarda) were only isolated from S2, whereas Staphylococcus intermidius was exclusive to S3 (Fig. 5). According to the overlap of species between months, S1vpresented four shared bacterial species for all the months of study, while only one species was common for all the studied months at S2 and S3.
Relationships between water characteristics and bacterial composition
The variability illustrated in the triplot of the redundancy analysis (RDA) totaled 44.5% of explained variance, with 27.6% on the first axis and 16.9% on the second axis (Fig. 6). The RDA revealed that the distribution of bacterial species that is projected on the same side of a given water quality parameter are positively influenced by the variation of this parameter, and inversely when this projection is on the opposite side. Accordingly, the phosphate, nitrates and magnesium, ammonium, sulphates positively affected the occurrence of Pseudomonas fluorescens, Pectobacterium carotovorum, Enterococcus faecalis, Klebseilla pneumoniae, Staphylococcus aureus, Cupriavidus necator, Plesiomonas shigelloides, and Staphylococcus epidermidis, especially in samples located at moderate-deep water. Moreover, TSS, DO, EC, and chloride significantly influenced the distribution of Enterobacter cloacae, Klebseilla oxytoca, Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, Pseudomonas aeruginosa, Pseudomonas putida, Pseudomonas syringe, Staphylococcus intermidius, Pantoea agglomerans, Morganella morganii, and Edwardsiella tarda, chiefly in shallow water samples. The distribution of bacterial species that is on the opposite side of a given water quality parameter is negatively correlated with that parameter. Bacterial species with close distributions to each other were similarly affected by water quality parameters (Fig. 6).
Tri-plot of the redundancy analysis showing the effects of water parameters on the distribution of bacterial species at different sampling sites in lake Tonga, northern Algeria (BOD5: biological oxygen demand, DO: dissolved oxygen, Dry.res: dry residues, EC: electrical conductivity, Hard.: hardness, TSS: total suspended solids, Turb: turbidity)
The water physicochemical parameters with significant effects and which were significantly correlated (p < 0.10) with the distribution of bacteria on the triplot of the RDA, were obtained with the first (27.6% of explained variance) and third axis (10% of explained variance) of RDA ordination (Table 2). On the first axis, the parameters that were significantly correlated were mainly phosphate (r = -0.72, p = 0.001) and magnesium (r = -0.49, p = 0.037), then ammonium (r = -0.41, p = 0.089), sulphate (r = -0.41, p = 0.095), and TSS (r = 0.40, p = 0.099). No parameter was correlated on the second RDA axis, however on the third axis, DO (r = -0.50, p = 0.033), EC (r = -0.62, p = 0.006) and nitrates (r = -0.54, p = 0.021), then TSS (r = -0.46, p = 0.056) and chloride (r = -0.42, p = 0.082) were significantly correlated.
Water quality index (WQI).
Values of WQI of water samples from Lake Tonga ranged between 67.7 and 147.2 (Fig. 7), indicating a water quality ranging from moderate quality to poor quality. Of the total samples analyzed, 44.4% had marginal water quality, whereas the remaining 55.6% samples had poor water quality. The lake water was of moderate quality (100 < WQI < 150) during January, February and March (cold period) for samples of S1 and S3, and during January and March for S2. The waterbody had poor quality (100 < WQI < 150) during April, May and June (hot period) at the three sites sampled as well as in February for S2. The variation in WQI values between months was significant (ANOVA: F(5,10) = 3.43, p = 0.046), but no significant variation was observed for the variation in WQI scores between the sampled sites (ANOVA: F(2,10) = 0.05, p = 0.951).
Determinants and spatiotemporal analysis of water quality
The spatial and temporal patterns of water quality appeared to be principally associated with physical and chemical variables, followed by bacterial groups. The double clustering heatmap (Fig. 8) showed that water samples exhibiting poor quality were consistently observed during the hot season, irrespective of the sampling site. Within these samples, the crucial physicochemical and bacteriological traits influencing water quality included chloride, dry residues, nitrates, EC, pH, TSS, and bacterial species richness. Subsequently, factors such as FC, TC, HTB, ammonia, and BOD5 also played significant roles.
A double clustering heatmap of physicochemical/bacteriological traits for different sampling months and sites in in Lake Tonga. All water parameters underwent standardization and scaling procedures. The clustering analysis employed the Ward method, with cluster distances determined by Euclidean distance. (Bacteria: number of species identified, THB: total heterotrophic bacteria, TC: total coliforms, FC: faecal coliforms, and FS: faecal streptococci; BOD5: biological oxygen demand, DO: dissolved oxygen, Dry.res: dry residues, EC: electrical conductivity, MH: magnesium hazard, TSS: total suspended solids)
Effect of WQI on bacterial species richness.
The effect of WQI on the variation in bacterial species richness in Lake Tonga was tested using a GLM for the entire study period, the cold rainy season as well as for the hot dry season (Fig. 9). The GLM revealed that the WQI had a negative influence (p = 0.030) on the variation in bacterial species richness during the cold rainy period, which means that during this period, the number of bacterial species decreased significantly with the increase in WQI values as the high species richness was observed in less polluted water samples. Though the relationship between WQI and species richness was positive during the hot dry season, the WQI did not seem to have a significant effect (p = 0.260) on the variation of species richness of water bacteria (Table 3).
Relationships between WQI and the variation of species richness of water bacteria for the entire study period (black line), cold rainy season (blue line) and hot dry season (red line) in Lake Tonga. Solid lines represent linear regressions obtained by Poisson GLM fitting with 95% confidence intervals. Circle size is mapped to the values of species richness
Effect of WQI on bacterial loads of contamination-indicators.
The effects of water quality index (WQI) on the variation of bacterial loads of the four groups indicators of fecal water contamination in Lake Tonga were tested using GLMs for the entire study period, the cold rainy season and for the hot dry season (Fig. 10). Based on GLM summaries (Table 4), the WQI showed a significant positive effect on the variation of the faecal streptococci bacterial group (p < 0.001) during the cold rainy period. For the entire study period, i.e. both cold-rainy and hot-dry seasons combined, the WQI positively affected the variation of the bacterial groups of THB (p = 0.023), TC (p = 0.020) and FC (p = 0.014). The WQI had no significant effects (p > 0.05) on the variation of cell loads of the bacterial groups THB, TC and FC during the cold rainy season and hot dry period taken separately (Fig. 10).
Scatter plot displaying the effect of WQI on the variation of water bacterial groups (THB: total heterotrophic bacteria, TC: total coliforms, FC: faecal coliforms, and FS: faecal streptococci) for the entire study period (black line), cold rainy season (blue line) and hot dry season (red line) in Lake Tonga. Solid lines represent linear regressions obtained by Poisson GLM fitting with 95% confidence intervals. Circle size is mapped to the values of species richness
Discussion
This study investigated the composition of bacterial communities in relation to the physicochemical characteristics and water quality of Lake Tonga, a wetland classified as a site of international importance and part of the biosphere reserve of El Kala National Park. Our results suggest that the highest bacterial species richness was found in shallow water (S1) with an average of 10.2 ± 0.8 species, while the lowest bacterial richness was found at deep lake water (S3) with an average of 7.8 ± 1.0 species. The first sampled site is located at the limit of the alder grove and is rich in aquatic plant species which can provide protection and optimal conditions to bacterial species, which are sensitive to predation and the harmful effects of UV rays. These conditions under this type of vegetation cover also provide abundant sources of nutrients from frond surface exudates (Devane et al. 2020). Badgley et al. 2010 demonstrated that the presence of vegetation in an aquatic habitat can significantly increase the number of Enterococci that may be able to persist throughout the habitat. Aquatic vegetation, in addition to serving as a substrate for the bacterial group, promotes high bacterial densities in the surrounding habitat. Interactions between aquatic vegetation and bacteria appear to be important phenomena that need to be better understood by biologists and microbial water quality managers (Cho et al. 2021).
The detection of high concentrations of Escherichia coli and Enterococci in the water indicates faecal contamination (WHO 2017). The results obtained by Loucif et al. (2020) After a quantitative estimation of the bacteria groups Indicators of faecal contamination, and an evaluation of the physico-chemical quality of the water revealed that there is significant pollution in Lake Tonga and the different faecal contamination indicator groups are linked to each other. In our study, Escherichia coli was noted in all samples. It is one of the commensal bacteria that make up the gut microbiota of humans and animals (Miquel et al. 2013; Yuan et al. 2018). Enterococcus feacalis have also been isolated, but with a lower relative abundance. In addition to Escherichia coli, several bacterial genera belonging to the total coliform group were identified (WHO 2017), namely the genus Klebsiella (Klebsiella oxytoca and Klebsiella pneumoniae), the genus Enterobacter (Enterobacter cloacae) and the genus Serratia (Serratia marcescens). In addition to coliforms and Enterococci, we have isolated and identified several bacterial species of medical, ecological and agricultural interest.
Staphylococci are bacteria involved in a variety of pathologies of varying degrees of severity. Staphylococcus aureus are one of the main agents responsible for nosocomial infections (Parija 2023). Its gram-positive bacteria are widely distributed and persistent in the environment, they can survive in adverse conditions such as low water activity, drought and even temperatures above 40 ºC (Faria et al. 2009; Valero et al. 2009). According to Valero et al. (2009), the optimum pH of Staphylococcus aureus growth was 6.5 and a slight decrease in the probability of growth was noted in the pH range of 7.0 to 7.5. Pseudomonas aeruginosa also poses major health problems, being highly opportunistic it represents a significant biotechnological and clinical burden (Qin et al. 2022). Given the wide distribution of Morganella morganii in nature, this bacterium can remarkably adapt and survive in different environmental conditions (Ghosh and LaPara 2007). Due to its rarity and low epidemic potential, little attention has been paid to this pathogen, but this bacterial species is now recognized as an important pathogen (Liu et al. 2016).
Fish pathogenic and phytopathogenic bacterial species have also been isolated; Edwardsiella tarda and Pseudomonas fluorescens are two bacterial species that are dangerous to pollution-sensitive freshwater fish (Das et al. 2019), Pseudomonas fluorescens readily proliferates in aquatic environments, including drinking water supply systems (Blancheton et al. 2013; Gołaś et al. 2019). According to Gołaś et al. (2019), an increase in NH4-N, NO3-N, BOD5 values was generally accompanied by an increase in the number of P. fluorescens determined by culture dependent and culture independent methods. The RDA revealed that NH4+, BOD5, and PO4, were positively correlated with P. fluorescens, especially in deep water. These parameters also contributed to the bacterial contamination of water. According to Li et al. (2020), P. fluorescens were negatively correlated with total nitrogen.
In the same context Pectobacterium carotovorum (formerly Erwinia) is one of the 10 most devastating plant pathogens and can cause significant economic losses in agricultural plant production during the growing season (Mansfield et al. 2012; Waleron et al. 2018). In Lake Tonga, we also identified Pseudomonas syringae, a species responsible for plant pathologies such as causing necrosis in black alder (Scortichini 1997; Lamichhane et al. 2014), an emblematic species of Lake Tonga. Diseases caused by P. syringae have been recognized as a major threat to the primary products of agroforestry (Lamichhane et al. 2014). Pseudomonas spp. are versatile and adaptable to different ecological niches. This adaptability explains their presence in a multitude of environments and their influence on agriculture and ecology (Mena and Gerba 2009; Dekak et al. 2020). Pseudomonas putida and Pseudomonas fluorescens are able to utilize multiple resources. Members of the P. putida group are more abundant. This may be due to differences in their interactions with the substrate, their interactions with other bacteria or the influence of bacterial density on the development of these microorganisms (Remold et al. 2015). Strains of the P. putida group are considered to be of low virulence, being found in both edaphic and aquatic environments and these colonize the roots of plants, creating a mutual relationship between the plant and the bacterium (Ogura et al. 2019).
Various species of microorganisms that make up the bacterial flora of Lake Tonga are among the bacteria that interact and contribute to the survival of vegetation. Pseudomonas syringae, P. fluorescens, Pantoea agglomerans are among the bacteria that solubilize phosphate and thus promote plant growth and yield (Karthikeyan et al. 2019). Cupriavidus necator is an important component of the nitrogen cycle in water; it is able to catalyze the reduction of nitrate to nitrite (Coelho et al. 2011) and Providencia rettgeri is a bacterium widely involved in the phenomena of heterotrophic nitrification, denitrification as well as in the process of transformation and elimination of nitrogen (Zhang et al. 2013). Ammonium elimination by P. rettgeri has also been reported to be strongly affected by oxygen availability and dependent on aerobic conditions (Taylor et al. 2009; Zhang et al. 2013). The reservoirs of the plesiomonads include estuaries and freshwater ecosystems, as well as the inhabitants of these areas. Plesiomonas shigelloides is an aquatic bacterium that typically resides in fresh and brackish waters in temperate and tropical climates (Janda et al. 2016).
The availability of nutrients as well as the rate of their diffusion can determine the strength of ecological competition between cells in a microbial community by reducing ecological competition (Nadell et al. 2010; Freilich et al. 2011). Competition between microbial cells plays an important role in the assembly of microbial communities (Mitri and Foster 2013). According to Qu et al. (2017), nutrients and sites within streams promote differences in microbial community composition. Thus, greater modularity in a bacterial community may be closely related to niche differentiation (Freedman and Zak 2015).
Indeed, the sites of water bodies that contain a high load of organic matter and receive more nutrients from the watershed provide rich niches for micro-organisms and contribute to the modelling of bacterial communities. The reduction of nitrate to ammonia by bacteria is a process that allows the conservation of nitrogen in a bioavailable form (NH4+), using nitrogen compounds and also provides energy (as ATP) to the microbes. A low level of NH4+ can stimulate certain N fixing bacteria. Nitrification is an essential process in the nitrogen cycle performed by nitrifiers that convert ammonium to nitrate (Qu et al. 2017). Freilich et al. (2011) demonstrated that cooperative interactions between bacteria are generally unidirectional and have no obvious benefit for the donor. However, within their natural communities, bacteria typically form tight cooperative loops, resulting in an indirect benefit for all species involved.
Anthropogenic land use increases nutrients and organic carbon in aquatic ecosystems and significantly controls the export of nutrients and organic matter from watersheds to waterbodies. This tightly controls the differences and diversity of microbial communities in these ecosystems (Qu et al. 2017). The composition of microbial communities can change in waterbodies under the influence of characteristics of land use of the watershed (Wang et al. 2011; Lear et al. 2013). Agricultural and urban activities increase concentrations of NO3− and NH4+ due to excessive application of fertilizers, as well as inputs from sewage and septic systems (Royer et al. 2004; Walsh et al. 2005; Allaoua et al. 2024). Increased nutrient load can lead to a significant decrease in water quality and thus alteration of aquatic communities (Justus et al. 2010; Loucif et al. 2020). The availability of N and P can limit the growth of autotrophic and heterotrophic bacteria (Tank and Dodds 2003; Johnson et al. 2009). The composition and diversity of bacterial communities in aquatic ecosystems change significantly with available nutrients (Fanta et al. 2010; Hill et al. 2011; van Horn et al. 2011; Drake et al. 2012). Potassium (K) is one of the essential elements for bacterial cells, naturally, soils contain more K than any other nutrient in a non-absorbable form, potassium solubilizing bacteria can convert it into soluble forms that can be absorbed by plants (Etesami et al. 2017).
Our findings showed that bacterial abundance was not positively associated with nutrient concentrations, such as phosphate, nitrates and magnesium. It is known that the bacterial abundance could be affected by mineral salts and nutrients (Steger et al. 2011) and phosphorus and nitrogen are necessary for the growth and metabolism of bacteria, however one or the other can be a limiting nutrient in some aquatic environments (Korajkic et al. 2019). In the case of Lake Tonga, the expansion of agricultural lands westward of the water body, combined with the use of fertilizers and sewage, septic tanks from settlements and populated villages near lake shores, have brought relatively more phosphate and nitrate and therefore bacterial abundance may be more limited by other factors when nutrients are abundant in the water (Luo et al. 2019). Our results revealed that bacterial abundances in water were negatively related to EC, this is consistent with results observed by He et al. (2007), which reported that high levels of EC in ponded water of the region of San Diego in Southern California provide a high concentration of salt inhibiting bacterial distribution or even damaging microorganisms. The primary production of THB tends to be limited under high P concentrations and/or high N:P ratio (Staley et al. 2016). Manini et al. (2004) also reported the limitation of THB distribution by phosphorus, and indicated that organic P can limit the growth of bacteria according to the hypothesis that the input of organic P by estuarine waters stimulates bacterial carbon production. However the extent of stimulation is greater when organic P supply is associated with decreasing organic N:P ratio.
We observed that the bacterial community distribution changes positively with DO concentrations. The DO content in waterbodies is an important variable in structuring communities, because it modifies the flow of energy in ecosystems. Oxygen is the main terminal electron acceptor, favoring the presence of aerobic respiration. Thus, THB groups use DO for the oxidation of organic matter. When oxygen is scarce, the next electron acceptor is nitrate (Aldunate et al. 2018). In addition, DO concentrations in Lake Tonga are low. In an aquatic environment when oxygen is depleted, there is a probability that the bacterial community will increase in taxonomic richness through the transfer of energy from the macrofauna to the microbes, as oxygen-dependent organisms avoid hypoxic areas or die (Baird et al. 2004; Spietz et al. 2015).
The WQI collects data from various regular water parameters and provides a value with a quick and understandable explanation of the water quality. The high values of the WQI in Lake Tonga correspond mainly to high values of EC, hardness, calcium, magnesium, nitrate, nitrite, ammonium, potassium, sulphates and chloride. These parameters contributed in a remarkable way to increase the value of WQI (Ustaoğlu et al. 2020; Chenchouni et al. 2023).
Our results indicated that the water of Lake Tonga coincides with the categories: moderate to poor water quality ranging from 67.7 to 147.2. Agricultural activities in the study area, the use of fertilizers, and direct discharge of untreated wastewater from neighboring towns have developed water pollution in this nature reserve (Loucif et al. 2020). These practices are common in natural wetlands and streams of North Africa (Belabed et al. 2017; Yalles-Satha et al. 2021), even in the surficial resources such as reservoirs destined for drinking water and irrigation (Bouaroudj et al. 2019; Negm et al. 2020a, b). The results obtained show a seasonal variation of WQI, lower WQI scores (average water quality) were generally recorded during the cold period, and higher scores (poor water quality) were recorded during the hot period. Seasonal rainfall and significant physical dilution of the surface waters of Lake Tonga would be the main cause of the variations observed in WQI values. A strong positive correlation of WQI was observed with THB, FC and TC present in the water of Lake Tonga of all the samples collected, as well as WQI affected the variation of FS (p < 0.001) during the cold period. The GLM revealed that WQI had a negative influence (p < 0.05) on the variation in bacterial species richness during the cold period.
According to Peruzzo et al. (2008) and Whitehead et al. (2009), the rainy season is characterized by a lower bacterial density compared to the warm and normal seasons, the heavy rainfall during the rainy period causes dilution of water bodies, this dilution within water bodies would be one of the important causes of seasonal variations in bacterial species abundance. In addition, the high densities of protozoa and metazoa that feed during the rainy season can decrease bacterial species richness by increasing mortality and decreasing nutrient concentration through consumption (Gurung et al. 2001). However, this finding contradicts the results of previous studies conducted in the South Nation River basin in eastern Ontario, Canada (Wilkes et al. 2009) and in the Göta älv River, Sweden (Tornevi et al. 2014), which indicated that the additional load from runoff was responsible for increased bacterial densities and pathogen detection after rainfall and that increased turbidity can affect the bacterial abundance in the water.
There is a need to focus on field studies to highlight the interactions between pathogens and the environment, as many studies on pathogen contamination of freshwater (rivers, lakes) have been carried out in the laboratory and some of the models introduced are expensive and have uncertainties, which can lead to poor decision making in water management and protection (Dean and Mitchell 2022). There is a need to develop new, efficient and accessible models and to improve the modelling approaches currently used to better predict the presence of pathogens in water. (Pandey et al. 2014; Dean and Mitchell 2022), to ensure the conservation of aquatic environments and their biodiversity, and to prevent further pollution especially in developing countries (Yarkwan 2023). In the framework of the results of the management and restoration plan of Lake Tonga elaborated by the Algerian Ministry of Agriculture and Rural Development, the constraints to the implementation of this management and restoration plan are particularly numerous and heavy (Benyacoub et al. 2011). Serious and lasting measures must be taken urgently; in particular, with regard to the discharge of wastewater, the urbanization of the watershed and the banks of the lake, and grazing. Many suggestions to remedy already polluted waters as well as to prevent further pollution have been drafted. since then, no action has been taken to preserve or restore the water quality of Lake Tonga. The problem is compounded by a lack of public awareness. Wastewater treatment is not a government priority and commitment to wastewater treatment and water quality improvement is extremely low.
Given the largely international dimension of the heritage value of Lake Tonga and for reasons of pollution and nuisance, water is the only mineral resource that can be considered for use. The only possible area of use of this water is agriculture. The quantities withdrawn must not jeopardize the optimal ecological functioning of the lake. The water must be taken away from the lake and must not be pumped in the immediate vicinity of the waterbody for reasons of pollution and noise. Furthermore, the agricultural use of water containing pathogenic bacteria constitutes a health risk for humans, hence the need to purify the water entering this aquatic ecosystem by building a collection and treatment system for domestic waste water, particularly on the side of Oued-El-Hout. Developing the sewerage systems of the settlements located in the catchment area of Lake Tonga can help in eliminating the system of non-drainable septic tanks located in the scattered rural villages.
Conclusion and perspectives
Lake Tonga is the most important freshwater site in the national Park of El-Kala, both in terms of area and ecological functions. This study analyzed the composition of the microbial community of the lake waters, which helped to identify an important cultivable bacterial flora whose specific richness varies according to water depths of the sites sampled and are also linked to the rhythm of the seasons. This microbial community, of which several species are potentially pathogenic for humans, animals and vegetation, is under the influence of water physical and chemical factors. Our analysis revealed that water physicochemical parameters are primary drivers of microbial community composition, diversity, and spatiotemporal distribution. The levels of nutrient salts recorded as well as the indicator germs of faecal contamination in Lake Tonga are quite important and confirm the state of eutrophication of the lake. The variations in physicochemical parameters have significant effects on the ecosystem by promoting oxygen-consuming microbial activity. For the proper ecological functioning of the region's wetlands, the conservation of this site is essential. It is therefore necessary to deploy the maximum of efforts to ensure that ecological functions and services of this wetland are sustained unscathed from any disturbance both at the level of the lake waterbody and at the lever of its watershed, it is recommended to (i) maintain the natural flows of ground and surface water, (ii) preserve good water quality of influents and put an end to any water pollution by untreated wastewater discharges from towns and dwellings bordering the lake, (iii) sensitize local populations on the ecological values, functions and ecosystem services of the site, and promoting benevolent and eco-responsible behavior, (iv) manage livestock grazing inside the wetland, and (v) put an end to the uncontrolled urban sprawl at the southern and western periphery of the lake. It is important to emphasis field studies and the relationship between water physico-chemical parameters and bacterial contamination in order to better understand the interactions of bacterial agents with the environment in surface freshwater systems. Few studies have focused on new models for predicting water contamination by potentially pathogenic bacterial agents. The improvement and/or the development of new models, as well as the integration of knowledge from different fields such as microbiology, hydrology and ecology, is necessary. The effect of WQI on the bacteriological quality of natural waterbodies is a very interesting topic to consider in modeling.
Availability of data and materials
The datasets used and analyzed during the current study are available from the authors on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BOD5:
-
5-Day biological oxygen demand
- CFU:
-
Colony-forming units
- DO:
-
Dissolved oxygen
- EC:
-
Electrical conductivity
- FC:
-
Faecal coliforms
- FS:
-
Faecal streptococci
- GLM:
-
Generalized linear model
- SD:
-
Standard deviation
- TC:
-
Total coliforms
- THB:
-
Total heterotrophic bacteria
- TSS:
-
Total suspended solids
- WHO:
-
World Health Organization
- WQI:
-
Water quality index
References
Aldunate M, de la Iglesia R, Bertagnolli AD, Ulloa O (2018) Oxygen modulates bacterial community composition in the coastal upwelling waters off central Chile. Deep Sea Res Part II 156:68–79. https://doi.org/10.1016/j.dsr2.2018.02.001
Alexander J, Hembach N, Schwartz T (2020) Evaluation of antibiotic resistance dissemination by wastewater treatment plant effluents with different catchment areas in Germany. Sci Rep 10:8952. https://doi.org/10.1038/s41598-020-65635-4
Allaoua N, Hafid H, Chenchouni H (2024) An appraisal of physicochemical and bacteriological quality of groundwater resources in semiarid lands of Algeria. J Arid Land 16(2):147–167. https://doi.org/10.1007/s40333-024-0004-4
Badgley BD, Thomas FI, Harwood VJ (2010) The effects of submerged aquatic vegetation on the persistence of environmental populations of Enterococcus spp. Environ Microbiol 12(5):1271–1281. https://doi.org/10.1111/j.1462-2920.2010.02169.x
Baird D, Christian RR, Peterson CH, Johnson GA (2004) Consequences of hypoxia on estuarine ecosystem function: energy diversion from consumers to microbes. Ecol Appl 14(3):805–822. https://doi.org/10.1890/02-5094
Belabed BE, Meddour A, Samraoui B, Chenchouni H (2017) Modeling seasonal and spatial contamination of surface waters and upper sediments with trace metal elements across industrialized urban areas of the Seybouse watershed in North Africa. Environ Monit Assess 189(6):265. https://doi.org/10.1007/s10661-017-5968-5
Belhouchet N, Inal A, Nait-Mohand H, et al. (2024) Assessment of water-sediment-biota pollution in coastal ecosystems of Algeria. Reg Stud Mar Sci 70:103355. https://doi.org/10.1016/j.rsma.2023.103355
Benyacoub S, Baba Hmed R, Kara H, Brahmia Z (2011) Plan Directeur de Gestion des Sites Ramsar du Parc national d’El Kala. Lac Tonga. Phase 1. T.A.D. Consultation for MADR, DGF, Algeria
BirdLife international (2020) Important bird areas factsheet: Lac Tonga. http://datazone.birdlife.org/site/factsheet/lac-tonga-iba-algeria
Blancheton JP, Attramadal KJK, Michaud L, d’Orbcastel ER, Vadstein O (2013) Insight into bacterial population in aquaculture systems and its implication. Aquac Eng 53:30–39. https://doi.org/10.1016/j.aquaeng.2012.11.009
Bouaroudj S, Menad A, Bounamous A, et al. (2019) Assessment of water quality at the largest dam in Algeria (Beni Haroun Dam) and effects of irrigation on soil characteristics of agricultural lands. Chemosphere 219:76–88. https://doi.org/10.1016/j.chemosphere.2018.11.193
Bouderbala A (2017) Assessment of water quality index for the groundwater in the upper Cheliff plain, Algeria. J Geol Soc India 90(3):347–356. https://doi.org/10.1007/s12594-017-0723-7
Boyd CE (2020) Water quality: an introduction. Third Edition. Springer, Switzerland. https://doi.org/10.1007/978-3-030-23335-8
Cardona C, Lax S, Larsen P, et al. (2018) Environmental sources of bacteria differentially influence host-associated microbial dynamics. mSystems 3(3):e00052-e118. https://doi.org/10.1128/msystems.00052-18
Chenchouni H, Chaminé HI, Khan MF, et al. (2022) New prospects in environmental geosciences and hydrogeosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-72543-3
Chenchouni H, Chaminé HI, Zhang Z, et al. (2023) Recent research on hydrogeology, geoecology and Atmospheric Sciences, Springer, Cham. https://doi.org/10.1007/978-3-031-43169-2
Cho KH, Wolny JL, Kase JA, Unno T, Pachepsky YA (2021) Interactions of E. coli with algae and aquatic vegetation in natural waters. Water Res 209:117952. https://doi.org/10.1016/j.watres.2021.117952
Coelho C, González PJ, Moura JG, Moura I, Trincão J, Romão MJ (2011) The crystal structure of Cupriavidus necator nitrate reductase in oxidized and partially reduced states. J Mol Biol 408(5):932–948. https://doi.org/10.1016/j.jmb.2011.03.016
Cotner JB, Biddanda BA (2002) Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5(2):105–121. https://doi.org/10.1007/s10021-001-0059-3
Das BK, Chakraborty HJ, Rout AK, Behera BK (2019) De novo whole transcriptome profiling of Edwardsiella tarda isolated from infected fish (Labeo catla). Gene 701:152–160. https://doi.org/10.1016/j.gene.2019.03.028
Dean K, Mitchell J (2022) Identifying water quality and environmental factors that influence indicator and pathogen decay in natural surface waters. Water Res 211:118051. https://doi.org/10.1016/j.watres.2022.118051
Dekak A, Menasria T, Benhizia Y, Chenchouni H (2020) Endophytic passenger bacteria associated with Genista cinerea nodules growing in north african drylands. Rhizosphere 14:100205. https://doi.org/10.1016/j.rhisph.2020.100205
Devane ML, Moriarty E, Weaver L, Cookson A, Gilpin B (2020) Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res 185:116204. https://doi.org/10.1016/j.watres.2020.116204
Dillon RJ, Vennard CT, Buckling A, Charnley AK (2005) Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8(12):1291–1298. https://doi.org/10.1111/j.1461-0248.2005.00828.x
Drake WM, Scott JT, Evans-White M, et al. (2012) The effect of periphyton stoichiometry and light on biological phosphorus immobilization and release in streams. Limnology 13(1):97–106. https://doi.org/10.1007/s10201-011-0359-z
D’Souza G, Shitut S, Preussger D, Yousif G, Waschina S, Kost C (2018) Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat Prod Rep 35(5):455–488. https://doi.org/10.1039/c8np00009c
Eckert EM, Di Cesare A, Kettner MT, Arias-Andres M, Fontaneto D, Grossart HP, Corno G (2018) Microplastics increase impact of treated wastewater on freshwater microbial community. Environ Pollut 234:495–502. https://doi.org/10.1016/j.envpol.2017.11.070
NF EN 872 (2005) T90–10. Qualité de l'eau-Dosage des matières en suspension–Méthode par filtration sur filtre en fibres de verre
NF EN ISO-15682 (2001) T90–082. Qualité de l'eau-Dosage des chlorures par analyse en flux (CFA et FIA) et détection photométrique ou potentiométrique
Etesami H, Emami S, Alikhani HA (2017) Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects a review. J Soil Sci Plant Nutr 17(4):897–911. https://doi.org/10.4067/S0718-95162017000400005
Fanta SE, Hill WR, Smith TB, Roberts BJ (2010) Applying the light: nutrient hypothesis to stream periphyton. Freshw Biol 55(5):931–940. https://doi.org/10.1111/j.1365-2427.2009.02309.x
Faria C, Vaz-Moreira I, Serapicos E, Nunes OC, Manaia CM (2009) Antibiotic resistance in coagulase negative Staphylococci isolated from wastewater and drinking water. Sci Total Environ 407(12):3876–3882. https://doi.org/10.1016/j.scitotenv.2009.02.034
Fishpool LD, Evans MI (2001) Important Bird Areas in Africa and associated islands: Priority sites for conservation. Cambridge, UK: BirdLife International.
Fox JW, McGrady-Steed J (2002) Stability and complexity in microcosm communities. J Anim Ecol 71(5):749–756. https://doi.org/10.1046/j.1365-2656.2002.00640.x
Freedman ZB, Zak DR (2015) Atmospheric N deposition alters connectance, but not functional potential among saprotrophic bacterial communities. Mol Ecol 24(12):3170–3180. https://doi.org/10.1111/mec.13224
Freilich S, Zarecki R, Eilam O, et al. (2011) Competitive and cooperative metabolic interactions in bacterial communities. Nat Commun 2(1):589. https://doi.org/10.1038/ncomms1597
Ghosh S, LaPara TM (2007) The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J 1(3):191–203. https://doi.org/10.1038/ismej.2007.31
Gołaś I, Szmyt M, Potorski J, et al. (2019) Distribution of Pseudomonas fluorescens and Aeromonas hydrophila bacteria in a recirculating aquaculture system during farming of European grayling (Thymallus thymallus L.) Broodstock. Water 11(2):376. https://doi.org/10.3390/w11020376
González-Cabaleiro R, Ofiţeru ID, Lema JM, Rodríguez J (2015) Microbial catabolic activities are naturally selected by metabolic energy harvest rate. ISME J 9(12):2630–2641. https://doi.org/10.1038/ismej.2015.69
Guemmaz F, Neffar S, Chenchouni H (2020) Physicochemical and bacteriological quality of surface water re-sources receiving common wastewater effluents in drylands of Algeria. In: Negm A, Bouderbala A, Chenchouni H, Barcelo D. Water Resources in Algeria-Part II water quality, treatment, protection and development. Springer Nature, Switzerland https://doi.org/10.1007/698_2019_400
Gurung TB, Kagami M, Yoshida T, Urabe J (2001) Relative importance of biotic and abiotic factors affecting bacterial abundance in Lake Biwa: an empirical analysis. Limnology 2(1):19–28. https://doi.org/10.1007/s102010170012
He LM, Lu J, Shi W (2007) Variability of fecal indicator bacteria in flowing and ponded waters in southern California: implications for bacterial TMDL development and implementation. Water Res 41(14):3132–3140. https://doi.org/10.1016/j.watres.2007.04.014
Hill WR, Rinchard J, Czesny S (2011) Light, nutrients and the fatty acid composition of stream periphyton. Freshw Biol 56(9):1825–1836. https://doi.org/10.1111/j.1365-2427.2011.02622.x
Janda JM, Abbott SL, McIver CJ (2016) Plesiomonas shigelloides revisited. Clin Microbiol Rev 29(2):349–374. https://doi.org/10.1128/cmr.00103-15
Johnson LT, Tank JL, Dodds WK (2009) The influence of land use on stream biofilm nutrient limitation across eight North American ecoregions. Can J Fish Aquat Sci 66(7):1081–1094. https://doi.org/10.1139/f09-065
Justus BG, Petersen JC, Femmer SR, Davis JV, Wallace JE (2010) A comparison of algal, macroinvertebrate, and fish assemblage indices for assessing low-level nutrient enrichment in wadeable Ozark streams. Ecol Ind 10(3):627–638. https://doi.org/10.1016/j.ecolind.2009.10.007
Kalinowska A, Jankowska K, Fudala-Ksiazek S, Pierpaoli M, Luczkiewicz A (2021) The microbial community, its biochemical potential, and the antimicrobial resistance of Enterococcus spp. in arctic lakes under natural and anthropogenic impact west Spitsbergen. Sci Total Environ 763:142998. https://doi.org/10.1016/j.scitotenv.2020.142998
Karthikeyan G, Rajendran L, Suganyadevi M, Raguchander T (2019) Microbial rhizobacteria-mediated signalling and plant growth promotion. Bioactive Molecules in Plant Defense, Springer, Cham, pp 35–59. https://doi.org/10.1007/978-3-030-27165-7_3
Kerr B, Riley MA, Feldman MW, Bohannan BJ (2002) Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature 418(6894):171–174.https://doi.org/10.1038/nature00823
Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF (2008) Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci 105(47):18188–18193. https://doi.org/10.1073/pnas.0807935105
Korajkic A, Wanjugi P, Brooks L, Cao Y, Harwood VJ (2019) Persistence and decay of fecal microbiota in aquatic habitats. Microbiol Mol Biol Rev 83(4):e00005-19. https://doi.org/10.1128/mmbr.00005-19
Lamichhane JR, Varvaro L, Parisi L, Audergon JM, Morris CE (2014) Disease and frost damage of woody plants caused by Pseudomonas syringae: seeing the forest for the trees. Adv Agron 126:235–295. https://doi.org/10.1016/B978-0-12-800132-5.00004-3
Lear G, Washington V, Neale M, Case B, Buckley H, Lewis G (2013) The biogeography of stream bacteria. Glob Ecol Biogeogr 22(5):544–554. https://doi.org/10.1111/geb.12046
Leung KY, Wang Q, Yang Z, Siame BA (2019) Edwardsiella piscicida: a versatile emerging pathogen of fish. Virulence 10(1):555–567. https://doi.org/10.1080/21505594.2019.1621648
Li P, Wu J, Qian H, Lyu X, Liu H (2014) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Ggeochem Health 36(4):693–712. https://doi.org/10.1007/s10653-013-9590-3
Li XM, Zhu YJ, Ringø E, Yang D (2020) Prevalence of Aeromonas hydrophila and Pseudomonas fluorescens and factors influencing them in different freshwater fish ponds. Iran J Fish Sci 19(1):111–124. https://doi.org/10.22092/ijfs.2019.120174
Liu Z, Huang S, Sun G, Xu Z, Xu M (2012) Phylogenetic diversity, composition and distribution of bacterioplankton community in the Dongjiang river, China. FEMS Microbiol Ecol 80(1):30–44. https://doi.org/10.1111/j.1574-6941.2011.01268.x
Liu H, Zhu J, Hu Q, Rao X (2016) Morganella morganii, a non-negligent opportunistic pathogen. Int J Infect Dis 50:10–17. https://doi.org/10.1016/j.ijid.2016.07.006
Logue JB, Lindström ES (2008) Biogeography of bacterioplankton in inland waters. Freshw Rev 1(1):99–115. https://doi.org/10.1608/frj-1.1.9
Loucif K, Neffar S, Menasria T, et al. (2020) Physico-chemical and bacteriological quality assessment of surface water at lake Tonga in Algeria. Environ Nanotechnol Monit Manag 13:100284. https://doi.org/10.1016/j.enmm.2020.100284
Loucif K, Maazi MC, Houhamdi M, Chenchouni H (2021) Nest site selection and breeding ecology of the ferruginous duck (Aythya nyroca) in Algeria. Global Ecol Conserv 26:e01524. https://doi.org/10.1016/j.gecco.2021.e01524
Loucif K (2020) Etude de la qualité physico-chimique et bactériologique de l’eau du lac Tonga (wilaya d’El Tarf) et occupation spatio-temporelle du site par l’avifaune aquatique. Doctoral thesis, Uni. Souk Ahras, Algeria. https://www.univ-soukahras.dz/fr/publication/article/2355
Luo X, Xiang X, Huang G, Song X, Wang P, Fu K (2019) Bacterial abundance and physicochemical characteristics of water and sediment associated with hydroelectric dam on the lancang river china. Int J Environ Res Public Health 16(11):2031. https://doi.org/10.3390/ijerph16112031
Manini E, Luna GM, Danovaro R (2004) Benthic bacterial response to variable estuarine water inputs. FEMS Microbiol Ecol 50(3):185–194. https://doi.org/10.1016/j.femsec.2004.06.011
Mansfield J, Genin S, Magori S, et al. (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13(6):614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Mena KD, Gerba CP (2009) Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol 201:71–115. https://doi.org/10.1007/978-1-4419-0032-6_3
Miquel S, Martin R, Rossi O, et al. (2013) Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 16(3):255–261. https://doi.org/10.1016/j.mib.2013.06.003
Mitri S, Foster KR (2013) The genotypic view of social interactions in microbial communities. Annu Rev Genet 47:247–273. https://doi.org/10.1146/annurev-genet-111212-133307
Nadell CD, Foster KR, Xavier JB (2010) Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol 6(3):e1000716. https://doi.org/10.1371/journal.pcbi.1000716
Negm A, Bouderbala A, Chenchouni H, Barcelo D (2020a) Water resources in Algeria - Part I: Assessment of surface and groundwater. Springer, Cham. https://doi.org/10.1007/978-3-030-57895-4
Negm A, Bouderbala A, Chenchouni H, Barcelo D (2020b) Water resources in Algeria - Part II: Water quality, treatment protection and development. Springer, Cham. https://doi.org/10.1007/978-3-030-57887-9
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75(1):14–49. https://doi.org/10.1128/mmbr.00028-10
Ogura K, Shimada K, Miyoshi-Akiyama T (2019) A multilocus sequence typing scheme of Pseudomonas putida for clinical and environmental isolates. Sci Rep 9:13980. https://doi.org/10.1038/s41598-019-50299-6
Oksanen J, Simpson G, Blanchet F et al (2022) Vegan: community ecology package. R package version 2.6–2. https://cran.r-project.org/web/packages/vegan/
Pandey PK, Kass PH, Soupir ML, Biswas S, Singh VP (2014) Contamination of water resources by pathogenic bacteria. AMB Express 4(51):1–16. https://doi.org/10.1186/s13568-014-0051-x
Parija SC (2023) Healthcare-associated infections. In: Parija SC (ed) Textbook of microbiology and immunology. Singapore, Springer-Nature, pp 1025–1038. https://doi.org/10.1007/978-981-19-3315-8_70
Peruzzo PJ, Porta AA, Ronco AE (2008) Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north Pampasic region of Argentina. Environ Pollut 156(1):61–66. https://doi.org/10.1016/j.envpol.2008.01.015
Qin S, Xiao W, Zhou C, et al. (2022) Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther 7(1):199. https://doi.org/10.1038/s41392-022-01056-1
Qu X, Ren Z, Zhang H, et al. (2017) Influences of anthropogenic land use on microbial community structure and functional potentials of stream benthic biofilms. Sci Rep 7:15117. https://doi.org/10.1038/s41598-017-15624-x
R Core Team (2022) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. R version 4.2.1. URL https://www.R-project.org
Rejsek F (2002) Analyse des eaux: aspects réglementaires et techniques. Collection biologie technique sciences et techniques de l’Environnement. Centre régional de documentation pédagogique (CRDP Aquitaine), Bordeaux
Remold SK, Purdy-Gibson ME, France MT, Hundley TC (2015) Pseudomonas putida and Pseudomonas fluorescens species group recovery from human homes varies seasonally and by environment. PLoS ONE 10(5):e0127704. https://doi.org/10.1371/journal.pone.0127704
Rodier J, Legube B, Marlet N (2009) L’analyse de l’eau, 9th edn. Dunod, Paris
Royer TV, Tank JL, David MB (2004) Transport and fate of nitrate in headwater agricultural streams in Illinois. J Environ Qual 33(4):1296–1304. https://doi.org/10.2134/jeq2004.1296
Sachs JL, Hollowell AC (2012) The origins of cooperative bacterial communities. Mbio 3(3):00099–00112. https://doi.org/10.1128/mbio.00099-12
Scortichini M (1997) Leaf necrosis and sucker and twig dieback of Alnus glutinosa incited by Pseudomonas syringae pv. syringae. Eur J Forest Pathol 27(5):331–336. https://doi.org/10.1111/j.1439-0329.1997.tb01087.x
Spietz RL, Williams CM, Rocap G, Horner-Devine MC (2015) A dissolved oxygen threshold for shifts in bacterial community structure in a seasonally hypoxic estuary. PLoS ONE 10(8):e0135731. https://doi.org/10.1371/journal.pone.0135731
Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ (2016) Sediments and soils act as reservoirs for taxonomic and functional bacterial diversity in the upper Mississippi river. Microb Ecol 71(4):814–824. https://doi.org/10.1007/s00248-016-0729-5
Steger K, Premke K, Gudasz C, Sundh I, Tranvik LJ (2011) Microbial biomass and community composition in boreal lake sediments. Limnol Oceanogr 56(2):725–733. https://doi.org/10.4319/lo.2011.56.2.0725
Tanentzap AJ, Fitch A, Orland C, et al. (2019) Chemical and microbial diversity covary in fresh water to influence ecosystem functioning. Proc Natl Acad Sci 116(49):24689–24695. https://doi.org/10.1073/pnas.1904896116
Tank JL, Dodds WK (2003) Nutrient limitation of epilithic and epixylic biofilms in ten north American streams. Freshw Biol 48(6):1031–1049. https://doi.org/10.1046/j.1365-2427.2003.01067.x
Taylor SM, Yiliang H, Bin Z, Huang J (2009) Heterotrophic ammonium removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Providencia rettgeri YL. J Environ Sci 21(10):1336–1341. https://doi.org/10.1016/S1001-0742(08)62423-7
Tornevi A, Bergstedt O, Forsberg B (2014) Precipitation effects on microbial pollution in a river: lag structures and seasonal effect modification. PLoS ONE 9(5):e98546. https://doi.org/10.1371/journal.pone.0098546
Ustaoğlu F, Tepe Y, Taş B (2020) Assessment of stream quality and health risk in a subtropical Turkey river system: a combined approach using statistical analysis and water quality index. Ecol Ind 113:105815. https://doi.org/10.1016/j.ecolind.2019.105815
Valero A, Pérez-Rodríguez F, Carrasco E, Fuentes-Alventosa JM, García-Gimeno RM, Zurera G (2009) Modelling the growth boundaries of Staphylococcus aureus: effect of temperature, pH and water activity. Int J Food Microbiol 133(1–2):186–194. https://doi.org/10.1016/j.ijfoodmicro.2009.05.023
van Elsas JD, Chiurazzi M, Mallon CA, et al. (2012) Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci 109(4):1159–1164. https://doi.org/10.1073/pnas.1109326109
van Horn DJ, Sinsabaugh RL, Takacs-Vesbach CD, Mitchell KR, Dahm CN (2011) Response of heterotrophic stream biofilm communities to a gradient of resources. Aquat Microb Ecol 64(2):149–161. https://doi.org/10.3354/ame01515
Waleron M, Misztak A, Waleron M, Franczuk M, Wielgomas B, Waleron K (2018) Transfer of Pectobacterium carotovorum subsp. carotovorum strains isolated from potatoes grown at high altitudes to Pectobacterium peruviense sp. nov. Syst Appl Microbiol 41(2):85–93. https://doi.org/10.1016/j.syapm.2017.11.005
Walsh CJ, Roy AH, Feminella JW, et al. (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24(3):706–723. https://doi.org/10.1899/04-028.1
Wang SY, Sudduth EB, Wallenstein MD, Wright JP, Bernhardt ES (2011) Watershed urbanization alters the composition and function of stream bacterial communities. PLoS ONE 6(8):e22972. https://doi.org/10.1371/journal.pone.0022972
Wang P, Wang X, Wang C, Miao L, Hou J, Yuan Q (2017) Shift in bacterioplankton diversity and structure: influence of anthropogenic disturbances along the Yarlung Tsangpo river on the Tibetan plateau. China Sci Rep 7:12529. https://doi.org/10.1038/s41598-017-12893-4
Whitehead PG, Wilby RL, Battarbee RW, Kernan M, Wade AJ (2009) A review of the potential impacts of climate change on surface water quality. Hydrol Sci J 54(1):101–123. https://doi.org/10.1623/hysj.54.1.101
WHO (2017) Guidelines for drinking-water quality: 4th ed. incorporating first addendum. Geneva: World Health Organization (WHO). https://www.who.int/publications/i/item/9789241549950
Wilburn P, Shchapov K, Theriot EC, Litchman E (2019) Environmental drivers define contrasting microbial habitats, diversity and functional redundancy in Lake Baikal, Siberia. Preprint bioRxiv. https://doi.org/10.1101/605899
Wilkes G, Edge T, Gannon V, et al. (2009) Seasonal relationships among indicator bacteria, pathogenic bacteria, Cryptosporidium oocysts, Giardia cysts, and hydrological indices for surface waters within an agricultural landscape. Water Res 43(8):2209–2223. https://doi.org/10.1016/j.watres.2009.01.033
Yalles-Satha A, Moutaouakil EAE, M, Kechemir L, Desvilettes C, Chenchouni H, (2021) Diversity, phenology and distribution of mayfly larvae (Ephemeroptera) along an altitudinal gradient in two permanent Wadis of Algeria. Orient inSects 56(1):14–46. https://doi.org/10.1080/00305316.2021.1904022
Yarkwan B (2023) Contamination of african water resources: impacts on biodiversity and strategies for conservation and restoration. In: Izah SC, Ogwu MC (eds) Sustainable utilization and conservation of africa’s biological resources and environment. Springer, Singapore, pp 469–495. https://doi.org/10.1007/978-981-19-6974-4_17
Yu H, Khan AU, Subramanian S, Vesper D, van Aken B (2019) Microbial communities in chesapeake Ohio canal national historical park and their function as indicators of water quality. Geomicrobiol J 36(8):673–682. https://doi.org/10.1080/01490451.2019.1612969
Yuan Y, Zheng G, Lin M, Mustapha A (2018) Detection of viable Escherichia coli in environmental water using combined propidium monoazide staining and quantitative PCR. Water Res 145:398–407. https://doi.org/10.1016/j.watres.2018.08.044
Zhang D, Li W, Huang X, Qin W, Liu M (2013) Removal of ammonium in surface water at low temperature by a newly isolated Microbacterium sp. strain SFA13. Biores Technol 137:147–152. https://doi.org/10.1016/j.biortech.2013.03.094
Zwart G, Crump BC, Kamst-van Agterveld MP, Hagen F, Han SK (2002) Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat Microb Ecol 28(2):141–155. https://doi.org/10.3354/ame028141
Acknowledgements
We thank Mr. Djamel Kahli (retired, former microbiologist at the hospital—EPH of El Kala) for his help in bacteriological analysis.
Funding
This study was not funded by any source.
Author information
Authors and Affiliations
Contributions
KL (Conceptualization; Methodology; Investigation; Data curation; Resources; Writing–original draft; Writing–reviewing & editing). HC (Supervision; Formal analysis; Visualization; Writing–original draft; Writing–reviewing & editing).
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interest.
Ethical Approval
This article does not involve any studies with human participants or animals conducted by any of the authors.
Consent to participate
The authors consent to participate in this research study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loucif, K., Chenchouni, H. Water physicochemical quality as driver of spatial and temporal patterns of microbial community composition in lake ecosystems. Appl Water Sci 14, 115 (2024). https://doi.org/10.1007/s13201-024-02176-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02176-5