- Review
- Open access
- Published:
Critical role of the gut microbiota in immune responses and cancer immunotherapy
Journal of Hematology & Oncology volume 17, Article number: 33 (2024)
Abstract
The gut microbiota plays a critical role in the progression of human diseases, especially cancer. In recent decades, there has been accumulating evidence of the connections between the gut microbiota and cancer immunotherapy. Therefore, understanding the functional role of the gut microbiota in regulating immune responses to cancer immunotherapy is crucial for developing precision medicine. In this review, we extract insights from state-of-the-art research to decipher the complicated crosstalk among the gut microbiota, the systemic immune system, and immunotherapy in the context of cancer. Additionally, as the gut microbiota can account for immune-related adverse events, we discuss potential interventions to minimize these adverse effects and discuss the clinical application of five microbiota-targeted strategies that precisely increase the efficacy of cancer immunotherapy. Finally, as the gut microbiota holds promising potential as a target for precision cancer immunotherapeutics, we summarize current challenges and provide a general outlook on future directions in this field.
Introduction
Microbes can be found throughout the human body, from external surfaces such as the conjunctiva, oral mucosa, and skin to internal surfaces such as the gastrointestinal tract and saliva. It has been estimated that trillions of bacteria, fungi, archaea, protozoa, and viruses exist throughout the body [1]. In accordance with this fact, there is also accumulating evidence that many physiological functions within the human body, including metabolism, inflammation, and the immune response, are influenced by microbes [2, 3]. Thanks to the technological boosts in large-scale sequencing over the past decade, multiple databases of the gut microbiome have been built to examine these functions(Table 1). These functions are related to the pathological processes of many human diseases, especially the development, progression, and immune evasion of cancer, as well as the modulatory effects of cancer treatments [4,5,6,7].
The essential properties of the gut microbiota, such as its stability, resilience, and diversity, need to be discussed, given its importance in human health [8]. The gut microbial community can be stable for years in healthy adults; thus, the microbiota has high stability. Homeostasis of the gut microbiota is maintained through negative feedback mechanisms [9]. The gut microbiota is often highly resilient to perturbations, thus allowing a host to maintain key species for long periods. However, understanding the resilience of this complex gut ecosystem is still challenging because the threshold for transitions of the gut microbiota to different states is only beginning to be determined [10, 11]. Microbial interactions ranging from mutualism and commensalism to competition and amensalism and the symbiotic relationship between microbes and their host can be considered essential factors in shaping gut stability and resilience of the gut microbiota [12]. With the recent advent of high-throughput sequencing, the diversity of the gut microbiota has been revealed at both the species and functional levels [13]. Functional screening by shotgun metagenomics contributes significantly to understanding the functional diversity of the gut microbiome. As more complementary “omics” datasets become available, functional variation in the gut microbiota in response to disease, diet, or other factors may be discovered [14]. For studies focusing on the diversity of the gut microbiota, a key challenge is understanding functional redundancy (i.e., which community species have similar functional niches and can substitute for one another). Funtional redundancy is also a critical aspect for conferring stability and resilience to the gut microbiota [15].
The gut microbiota has been shown to play critical roles in maintaining intestinal barrier integrity and homeostasis. The composition of the gut microbiome is under the surveillance of the intestinal immune system. Inflammation caused by an imbalance between commensal and pathogenic microbes can lead to intestinal and even systemic diseases [16]. In terms of the mutually beneficial symbiotic ecosystem between the gut microbiota and the host, the host offers habitats and nutrients in the gut, while the microbes support the maintenance of lipid and glucose metabolism and the maturation of the intestinal immune system by providing microbiome-derived metabolites [17]. For instance, short-chain fatty acids (SCFAs), including acetic acid, butyric acid, and propionic acid, are essential energy sources for gut microbes and perform diverse regulatory functions related to host physiology and immunity [18]. Trimethylamine N-oxide (TMAO), which is a molecule generated from gut microbial metabolism, is also associated with host immunity [19].
Current research on the relationship between cancer and microbes has mostly focused on the gut microbiota and demonstrated a complicated interaction between the gut microbiota and the immune system; this interaction was evaluated by determining the composition of the gut microbiota [20]. For example, observations of developmental defects in germ-free (GF) mice suggest that systemic immune function may be impaired in the absence of the gut microbiota [21]. Moreover, the gut microbiota and its metabolites have been proposed to be critical factors involved in modulating the efficacy and toxicity of cancer immunotherapy. A landmark example was presented by Sivan et al. [22], who first reported the complicated crosstalk between the gut microbiota and programmed cell death protein-1 (PD-1)/PD-1-ligand 1 (PD-L1) blockade.
Consistent with the demonstrated relationships between the gut microbes and immune responses, many in vitro and in vivo studies have also noted a promising approach for optimizing the therapeutic outcomes of cancer immunotherapy: manipulating the composition of the gut microbiota [23, 24]. However, although the concept of using the gut microbiota as a tool for precision medicine has developed rapidly over the last decade [25], the number of published studies exploring practical interventions to modify the gut microbiota is rather limited and unspecific. In this review, we will discuss five commonly explored interventions that have had relatively strong impacts on the therapeutic outcomes of cancer immunotherapy, namely, fecal microbiota transplantation (FMT), diet, probiotics, prebiotics, and engineered microbial products. Compared with the other four methods, FMT is a well-established clinical approach recommended by the FDA for modulation of the gut microbiota. The gut microbes from a healthy host are transplanted to recover microbial homeostasis in the recipient. However, the research has been restricted to correlation relationships rather than causality, and outlining the future direction of clinical applications utilizing the gut microbiota is challenging. With multiomics tools and synthetic biology, we can now explore the exact mechanism underlying gut microbiota modification in cancer immunotherapy. Here, we will also provide evidence to support the incorporation of gut microbiota modification in immunotherapy while acknowledging the challenges in this rapidly developing field.
The interplay between the immune system and the gut microbiota
Gut microbiota symbiosis plays a multifaceted role in shaping the immune responses of the human host [26, 27]. This complicated crosstalk allows for the normal functioning of immune tolerance and immunosurveillance, which recognizes and eliminates opportunistic bacteria to prevent potential infection. The critical role of the gut microbiota in the formation of a fully functional immune system was identified in GF animals [28]. As a go-to animal model for bacteria-host interactions, GF animals display distinct features in the gut, including an immature mucus system, unformed gut-associated lymphoid tissues, and a reduced number of immune cells [29,30,31,32,33]. Here, we summarize the current views on how the gut microbiota influences various components of the systemic immune system. We roughly divided the following discussion into three parts: non-gastrointestinal (GI) tract lymphoid organs, the innate immune system, and adaptive immune system components in the GI tract. Specifically, we summarize the interactions between immune cells and gut microbiota (Table 2).
Lymphoid organs
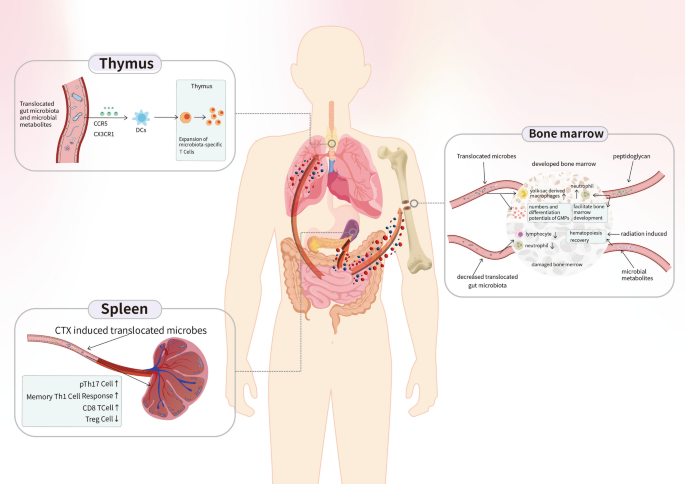
Regarding the interplay of non-GI tract lymphoid organs with the gut microbiome, several studies have revealed immunological modulation by microbes in the thymus, bone marrow, and spleen. Initial clinical evidence showed an association between primary lymphoid organs and the gut microbiota in patients with hematologic malignancies [34, 35]. This association was further validated with mouse models by Staffas et al. [36], where depletion of the gut microbiota led to significant reductions in lymphocyte and neutrophil counts. Moreover, metabolites such as SCFAs can facilitate the recovery of hematopoiesis in bone marrow after radiation damage [37]. The developed bone marrow can work together with translocated gut microbiota to drive the expansion of yolk sac-derived macrophages, increase the number of granulocytes and monocyte progenitors, and promote their differentiation [38]. In addition, bone marrow development can also be affected by peptidoglycans, which modulate neutrophil function [39]. In the thymus, studies have demonstrated that recolonization of the gut microbiota drives the thymic expansion of T cells. Specifically, the gut microbiota is trafficked to the thymus in a CX3CR1- and CCR5-dependent manner by intestinal CX3CR1 DCs, which assist in inducing the expansion of microbiota-specific T cells [40]. Researchers have demonstrated that cyclophosphamide (CTX) induces the translocation of selected bacteria into the spleen, followed by the stimulation of a specific subset of “pathogenic” helper T (Th) 17 cells, which generate memory Th1 immune responses and increase the CD8 + /Regulatory T(Treg) cell ratio [41, 42] (Fig. 1).
The interplay between the immune system and the gut microbiota in non-GI tract lymphoid organs. The gut microbiota and its metabolites influence the development of host bone marrow and thymus. For instance, SCFAs are capable of facilitating hematopoiesis recovery of bone marrow after radiation damage.The gut microbiota also induce the translocation of selected bacteria into and stimulate immunocytes and immune responses of the spleen after CTX treatment
Antimicrobial peptides (AMPs)
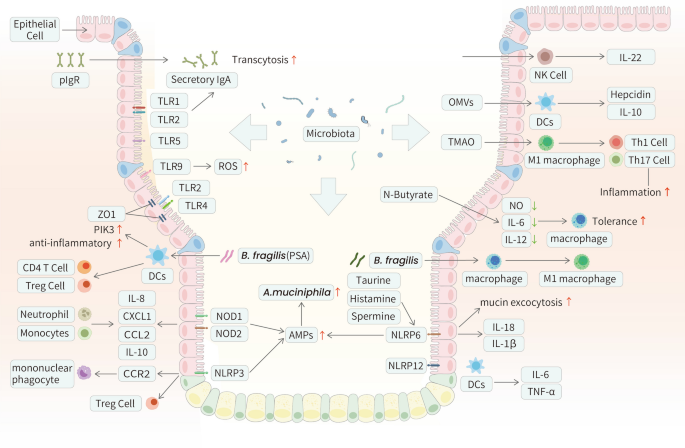
AMPs are secreted by epithelial cells in the gut, mostly Paneth cells [43]. They are a crucial component of immunoreactive substances, and affect the innate immune system. As the first-line defender, AMPs modulate the immune system in response to a wide range of invasive pathogens. The most abundant AMPs, human defensin(HD) HD-5 and HD-6, modulate the microbiota in vivo via an increase in the abundance of Akkermansia sp [44]. In mouse models, the lack of pore-forming Orai1 was associated with high mortality due to severe intestinal bacterial dysbiosis, and the absence of AMP secretion from acinar cells was considered the major cause [45] (Fig. 2).
The interplay between the innate immune system and the gut microbiota in GI tract. Some mechanisms utilized by the gut microbiota to interact with the host innate immune system in GI tract are described above. The interplay between the gut and its microbiota is complex. The secretion of AMPs could be affected by A.muciniphila. PRRs are strongly affected by the presence of the gut microbiota. Microbiota-derived TLR and NOD ligands act directly on intestinal immunocytes and can activate inflammatory genes. Bacteroides fragilis stimulates the downstream PI3K pathway and activates the transcription of anti-inflammatory genes by co-operating TLR1/TLR2 heterodimer and Dectin-1. NLRs function to activate inflammatory caspases and cytokines to compost optimal microbiota and maintain intestinal homeostasis. Microbial metabolites taurine, histamine, and spermine have been identified to regulate the activation of NLRP6 inflammasome as well as the induction of downstream epithelial IL-18 and AMPs secretion. Innate immune cells, including macrophages, DCs, and NK cells, interact heavily with the gut microbiota. OMVs derived from Bacteroides elicit IL-10 production by DCs, as well as enhance the phagocytic functions of macrophages triggered by the bacteria themselves. The expression of the transcription factor RORγt and IL-22 of intestinal NK cells is conditioned by the commensal microbiota
Pattern recognition receptors (PRRs)
PRRs identify host receptors that recognize specific pathogen-associated molecular patterns (PAMPs), making PRRs a critical factor in defense against infectious pathogens [46]. Following activation by PAMPs, PRR signaling pathways produce AMPs, cytokines, chemokines, and apoptotic factors. These factors are expressed not only in innate immunity but also in various nonprofessional immune cells, such as intestinal epithelial cells (IECs) in the GI tract. The most well-studied PRRs are toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs) [47]. Understanding how microbes influence PRR-associated immune responses is fundamental for understanding gut microbiome homeostasis.
TLRs are widely expressed in the GI tract but differ significantly between the intestine and colon [48]. We focused on TLR4, TLR5, TLR9, and TLR2, which are involved in microbe recognition. In the context of the GI tract, TLR2 is expressed in mononuclear cells of the lamina propria, goblet cells, and enterocytes. TLR4 and TLR9 are expressed mainly in IECs [49]. In addition, TLR5 is expressed on the basolateral side of IECs in the colon, while its expression is restricted to Paneth cells in the small intestine [50]. TLRs are strongly affected by the presence of microbes [51]. In particular, we will discuss how TLR signaling mediates the crosstalk between microorganisms and IECs and how this structural and functional interplay primes immune cell responses in the gut mucosa. Microbial metabolites strongly regulate IEC proliferation, apoptosis, and differentiation [52]. These processes can be induced by the development of goblet cells that are activated by TLR2 and TLR4 [53]. The motility of intestinal smooth muscle could be another factor that impacts the differentiation of IECs, which is mediated by TLR4, TLR5, and TLR9 [54, 55]. Researchers have revealed that TLR2 stimulation effectively preserves tight junction-associated barrier integrity by promoting phosphoinositide 3-kinase (PI3K)/Akt-mediated cell survival via myeloid differentiation primary response gene 88 (MyD88) as well as the translocation of zona occludens 1 (ZO1) and occluding proteins [56]. Moreover, activation of TLR4 induces a loss of barrier function through the expression of myosin light chain kinase (MLCK) [57]. In addition, AMP and IgA transcytosis are highly dependent on TLR-mediated recognition of the gut microbiota [58, 59]. IECs control microbial invasion of the mucosa through the release of ROS into the lumen after TLR activation [60]. These results indicate that TLRs are involved in intercellular junctions, and that enhancing or disrupting intestinal epithelial barrier integrity depends on microbes. A typical example for understanding TLR–microbe interplay is the symbiont molecule polysaccharide A (PSA) of Bacteroides fragilis (B.fragilis). PSA interacts with the TLR1/TLR2 heterodimer on DCs in cooperation with Dectin-1 to stimulate the downstream PI3K pathway, followed by the transcription of anti-inflammatory genes. This PSA-dependent immunomodulation is essential for presenting CD4 + T cells and Treg cells, which are critical for producing interleukin-10 (IL-10), which is the primary anti-inflammatory outcome [61, 62].
NLRs activate inflammatory caspases and cytokines and modulate inflammatory signaling pathways [63]. NOD1/NOD2 recognizes peptidoglycan in bacterial cells and activates the NF-κB/extracellular-signal-regulated kinase(ERK) /mitogen-activated protein kinase(MAPK) signaling pathway to mediate cytokine, chemokine, and antimicrobial peptide expression, thereby promoting the host immune response [64,65,66]. Specifically, stimulation of epithelial cells with NOD1 stimulatory molecules can induce the production of CXCL1, CCL2, IL-8, and AMPs, which are essential for recruiting neutrophils [67]. In NOD2(-/-) mice, inflammatory pathologies associated with the expansion of Bacteroides vulgatus were observed [68]. Researchers confirmed that NOD2 mediates CCL2-CCR2-dependent recruitment of inflammatory monocytes and promotes their production of IL-10 [69]. Moreover, the anti-inflammatory effects of Lactobacillus salivarius Ls33 were abrogated in NOD2(-/-) mice [70]. NOD-like receptor thermal protein domain associated protein(NLRP)3, plays a well-defined role in intestinal homeostasis and protection against inflammation [71]. According to Seo et al. [72], Proteus mirabilis (P. mirabilis) can induce robust IL-1β release by meditating the recruitment of CCR2 mononuclear phagocytes. Similarly, Yao et al. [73] confirmed that the hyperactive NLRP3 inflammasome could remodel the gut microbiota by inducing IL-1β production. Furthermore, they observed enhanced production of AMPs and compensatory changes in local Treg cell levels to neutralize inflammation. Another well-studied inflammasome-forming NLR is NLRP6. Elinav et al. [74] described the novel regulatory mechanism of the NLRP6 inflammasome in which a deficiency of NLRP6 resulted in reduced IL-18 and IL-1β levels. Additionally, NLRP6 knockout mice had an increased abundance of Akkermansia muciniphila (A.muciniphila) [75]. Wlodarska et al. [76] further explored the regulatory effect of the NLRP6 inflammasome on the biogeographical distribution of the gut microbiota, and the authors suggested that NLRP6 mediates mucin granule exocytosis and subsequent mucous layer formation. In another study, Levy et al. [77] reported that taurine, histamine, and spermine activated NLRP6 inflammasome and induced downstream epithelial IL-18 and AMP secretion. In addition to inflammasome formation, NLRP12 suppresses NF-κB signaling and the expression of downstream inflammatory cytokines [78,79,80,81]. Two recent studies have connected NLRP12 with the gut microbiota in the contexts of colon inflammation and obesity. Chen et al. [82] found that microbial dysbiosis contributed to colitis in NLRP12 knockout mice. These mice exhibited increased expression of inflammatory cytokines, including tumor necrosis factor-α(TNF-α) and IL-6, by DCs, which was reversed by the administration of Lachnospiraceae. In addition, inflammation associated with obesity in NLRP12-deficient mice was attributed to the maintenance of beneficial microbiota [83] (Fig. 2).
Macrophages
Macrophages are known as the first-line of defense against pathogens, but they also interact heavily with commensal bacteria [84]. B. fragilis enhances the phagocytic functions of macrophages by polarizing them to an M1 phenotype [85]. Researchers have shown that the gut microbiota promotes the interaction between IL-1β–secreting macrophages and colony-stimulating factor 2 (Csf2)-producing RORγt + innate lymphoid cells 3 (ILC3s) [86]. Several studies have explored the influence of microbial products on macrophages. By inhibiting the release of NO, IL-6, and IL-12, n-butyrate may assist in the tolerance of colon macrophages to commensals [87]. Furthermore, butyrate-enhanced antimicrobial activity was shown to be related to alterations in macrophage metabolism and increased LC3-associated antimicrobial clearance [88]. TMAO-polarized inflammatory macrophages induce a potent Th1 and Th17 response by modulating the microenvironment, which exacerbates inflammation-related diseases [89] (Fig. 2).
Dendritic cells (DCs)
DCs are the most potent and versatile professional antigen-presenting cells (APCs), that can initiate the adaptive immune response and support innate immunity [90]. DCs can be divided into plasmacytoid DCs (pDCs) and conventional DCs (cDCs) [91, 92]. Researchers have suggested that cDCs cannot be fully activated due to insufficient interferon-I(IFN‐I) signaling. In other words, the gut microbiota, which is the major regulator of IFN-I secreted by pDCs, controls the basal state of DCs [93]. Another example of this crosstalk is the outer membrane vesicles (OMVs) derived from Bacteroides thetaiotaomicron. These OMVs are instrumental in eliciting regulatory IL-10 production by DCs [94]. In addition, Bessman et al. [95] reported that hepcidin produced by cDCs in response to microbiota-derived signals promoted intestinal homeostasis. (Fig. 2).
Natural killer (NK) cells
NK cells are an important component of the innate immune system and account for up to 15% of all lymphocytes [96]. Researchers have suggested that the innate mucosal defense provided by a subset of intestinal NK cells is conditioned by the commensal microbiota, which expresses the transcription factors RORγt and IL-22 [97]. Four trials applying synbiotics or probiotics have shown that administration improved the gut microbiota composition and increased NK cell activity and the levels of associated cytokines [98,99,100,101]. More specifically, Qiu et al. [102] reported that the probiotic Lactobacillus plantarum can efficiently increase the expression of IL-22 mRNA and protein in NK cells, thereby mitigating intestinal epithelial barrier damage. (Fig. 2).
B cells
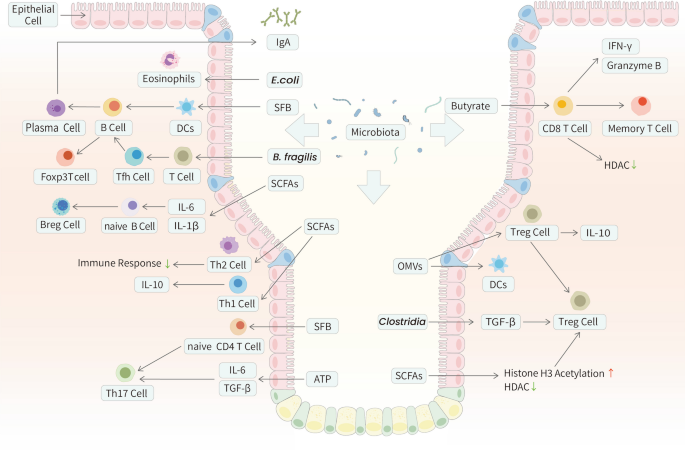
B cells are crucial mediators of intestinal homeostasis. By secreting immunoglobulins and cytokines, they assist in maintaining a noninflammatory host-microbe relationship [103, 104]. GF mice show a reduced amount of immunoglobulin A, a differentiated form of B-cell, and impaired B-cell responses [105]. The intestinal colonization of E. coli, bifidobacteria, and segmented filamentous bacteria (SFB) might promote B-cell maturation and enhance the specific IgA antibody response [106, 107]. This IgA response helps maintain gut microbiota homeostasis, thereby facilitating the expansion of Foxp3 + T cells and maturation of the gut immune system through a symbiotic regulatory loop [108]. The regulation of B cells by the gut microbiota and its products could be influenced by IgA, immune cells, chemokines, cytokines, or even B cells themselves [109]. More specifically, B-cell activating factors can be induced by IECs, DCs, T cells, and eosinophils. Together, these immune cells and cytokines can promote the differentiation and survival of IgA plasma cells [110,111,112,113,114]. Additionally, microbial metabolites such as SCFAs activate B-cell receptors (BCRs), inhibit histone deacetylases (HDACs), and increase adenosine triphosphate (ATP) levels [115, 116]. The differentiation of naïve B cells into regulatory B cells (Bregs) can be induced by intestinal microbiota-driven production of IL-1β and IL-6 [117] (Fig. 3).
The interplay between the adaptive immune system and the gut microbiota in GI tract. Some mechanisms utilized by the gut microbiota to interact with the host innate immune system in GI tract are described above. Foxp3 + Treg cells promote maturation of B cells and production of secretary IgA. These contribute to the regulation of homeostatic microbiota composition and the maintenance of a non-inflammatory host-microbial relationship. CD8 + T cells can be activated by the intestinal microbiota and its metabolites. Butyrate, for instance, showed a direct antagonistic influence on the HDAC of CTLs and Tc17 cells, thereby promoting the expression of IFN-γ and granzyme B. As for Th cells, the adhesion of SFB to IECs is a common outcome of inducing homeostatic intestinal Th17 cells. Tfh cells, being another modulation target of gut microbiota modification, are essential for the production of plasma cells and memory B cells. The SCFAs have been demonstrated to regulate the size and function of the Treg cell pool
CD8 + T cells
T cells coordinate the immune response and directly kill damaged cells. These functions are mediated by CD4 + and CD8 + T cells, respectively. CD8 + T cells play central roles in controlling infections and cancer. These cells are known to secret IFN-γ and the protease granzyme B, which act synergistically to kill infected or tumorigenic cells [118]. CD8 + T cells can be activated by the intestinal microbiota and its metabolites, such as cytotoxic T lymphocytes (CTLs), to exert direct cytotoxicity and interact with other immune cells, especially in the tumor microenvironment (TME) [119]. Conversely, microbial dysbiosis exacerbates chronic inflammation and tumor susceptibility, thereby attenuating the activity of CD8 + T cells and sometimes even causing their exhaustion [120,121,122,123]. Moreover, butyrate had a direct antagonistic influence on the HDACs of CTLs and cytotoxic T lymphocyte 17 (Tc17) cells, thereby promoting the expression of IFN-γ and granzyme B [124]. Butyrate could also promote activated CD8 + T cell differentiation into memory cells [125]. Immunotherapy targeting the close interaction between CD8 + T cells and the gut microbiota is promising and will be discussed below (Fig. 3).
Helper T (Th) cells
Th cells, which are differentiated from naïve CD4 + T cells, can orchestrate humoral and cellular immunity by facilitating the activation of immunocytes in a cytokine-dependent manner [126, 127]. Different subsets of Th cells show distinct functions in protective immunity and reactivity to the gut microbiota because of differences in the production of signature cytokines [128]. Th1 cells produce IFN-γ, IL-2, and TNF-α, and the expression of IL-4, IL-5, and IL-13 defines Th2 cells. Th17 cells are abundant within the GI tract and help regulate gut microbes. The signature cytokines of this cell subset include IL-17A, IL-17F, and IL-22 [129]. Th1 and Th2 cells exhibit functions that are regulated by the gut microbe-derived metabolites [130]. SCFAs are associated with an impaired ability to initiate a Th2 cell immune response [131]. Additionally, SCFAs can promote microbe antigen-specific IL-10 production in Th1 cells through GPR43 and induce the expansion of the Th1 transcription factor T-bet [132]. Furthermore, cancer patients display decreased plasma tryptophan(Trp) levels correlated with an increase in Th1-type immune activation markers [133]. The potential association between Th17 cells and gut microbes has been shown in different diseases. Specific alterations in the intestinal mucosa-associated microbiota were correlated with an increased number of intestinal Th17 cells and a high disease burden [134]. Preclinical models further verified this correlation by showing that augmenting the population of pathogenic colonic Th17 cells could promote tumorigenesis [135]. However, their causal relationships have not been proven. We propose that the delicate balance of plasticity makes Th17 cells potential pathogenic drivers of intestinal immune diseases [136,137,138,139,140,141]. Studies have shown that the gut microbiota and metabolites activate Th17 cells. The impaired plasticity of Th17 cells in the absence of the gut microbiota can be restored by microbial metabolites [142,143,144]. SFB is a representative example of a molecule that can induce homeostatic intestinal Th17 cells [145, 146]. Atarashi et al. [147] further demonstrated that the adhesion of SFB to IECs is a critical factor for inducing Th17 cells and antigen binding to pro-Th17 DCs. Another study revealed that Bifidobacterium adolescentis could influence Th17 cells in a similar manner [148,149,150]. Researchers have shown that ATP derived from commensal bacteria can activate a unique subset of lamina propria cells, namely, CD70high/CD11clow cells, which induce IL-6 and transforming growth factor(TGF)-β, leading to the differentiation of Th17 cells [151]. Moreover, different gut microbe-derived BA and SCFA metabolites regulate and modulate Th17 cell immunological function and differentiation [152, 153]. Various diets have also been shown to have complicated impacts on Th17 cells [154, 155] (Fig. 3).
Follicular helper T (Tfh) cells
Another critical subset of Th cells is Tfh cells. In addition to assisting B cells in producing antibodies, Tfh cells are essential for germinal center (GC) formation, affinity maturation, and the production of memory B cells [156]. The maturation of Tfh cells is restricted in GF mice, resulting in diminished IgA development and disruptions in microbial homeostasis [111]. Alterations in the gut microbiota can be observed in Tfh cells when ATP-gated ionotropic P2X7 receptors are absent [157, 158]. Moreover, bacteria of the genus Anaeroplasma can increase intestinal IgA levels by inducing TGF-β in Tfh cells [159] SFB can induce the differentiation of Tfh cells and egress into systemic sites, thereby facilitating systemic Tfh cell responses and autoantibody secretion that can worsen diseases [160]. Microbiota-derived eATP can also regulate Tfh cell abundance [161]. Thus, the gut microbiota can be a modulatory target of Tfh cells to further impact intestinal immunity [162] (Fig. 3).
Some Treg cells are also found in B-cell follicles and were identified as T follicular regulatory (Tfr) cells. These cells can migrate into the GC, thereby inhibiting B-cell maturation and antibody production [163] SFB, which induces Tfh cells to promote autoimmune arthritis, has also exhibited the potential to influence systemic Tfr cells [164]. In addition, butyrate is an environmental cue that can induce the differentiation of Tfr cells, which can also ameliorate autoimmune arthritis [165].
Regulatory T (Treg) cells
Treg cells, which differentiate from naïve CD4 + T cells, are an irreplaceable constituent of immunity and are involved in the maintenance of immunological self-tolerance and homeostasis. Treg cells express the transcription factor Foxp3 in the nucleus and CD25 and CTLA-4 on the cell surface [166]. These factors are modulated by gut microbial signals [167,168,169,170]. TGF-β, the physiological inducer of the transcription factor Foxp3 (associated with the development of Treg cells), can be induced by Clostridia [171, 172] B. fragilis has been shown to form OMVs, packed with capsular PSA, and increase IL-10 expression in Treg cells, and activate TLR2 ligation on T cells and DCs [173, 174]. SCFAs have been demonstrated to regulate the size and function of the Treg cell pool [175, 176]. Specifically, butyrate promotes histone H3 acetylation at the Foxp3 locus, and propionate inhibits HDACs [177, 178].
In summary, microbes exert positive and negative effects on the immune system of the GI tract, thus indicating their dual role in cancer progression. Gut microbiome homeostasis enhances the host immune response. However, dysbiosis and depletion of the gut microbiome interfere with the immune system abnormally by manipulating various innate and adaptive immune system components, which may further increase susceptibility to tumorigenesis. (e.g., inducing a loss of intestinal barrier function through the PRR signaling pathway; affecting B-cell differentiation and response; attenuating CD8 + T cells, even causing their exhaustion; causing impaired plasticity in Th17 cells; and restricting the maturation of Tfh cells). Specifically, different strains of gut microbes play different roles in regulating GI tract immunity. In the GI tract, A.muciniphila, B.fragilis, Ls33, Lachnospiraceae, E. coli, bifidobacterial, SFB, and Bifidobacterium adolescentis are associated with immune cell activation processes and exhibit anti-inflammatory properties. Moreover, strains like Bacteroides vulgatus displayed inflammatory pathologies, which might be involved in cancer progression. Microbial metabolites showed similar dual characteristics. Butyrate attenuates the inflammatory response, while TMAO promotes it.
The gut microbiota and the efficacy of cancer immunotherapy
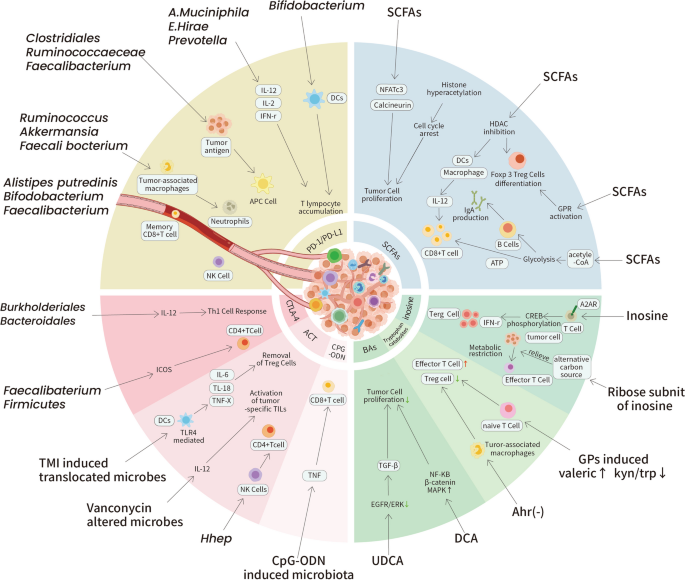
The idea of cancer immunotherapy has evolved rapidly in the past few decades. Many types of immunotherapy have been developed to revive the immune system by suppressing the immunoinhibitory pathways commonly employed by tumor cells to escape immunosurveillance. A close link between the gut microbiota and cancer immunotherapy has slowly been unveiled with an increasing number of innovative studies. We outline the recent evidence in this field by type of immunotherapy (Additional file 1: Table S1) (Fig. 4).
Selected mechanisms of how the gut microbiota impact cancer immunotherapies. Current studies have revealed the close link between the gut microbiota and the efficacy of cancer immunotherapy. Grouped by immunotherapies and metabolites, outlined here are some selected mechanisms utilized by the gut microbiota and its metabolites to regulate immunocyte activation, cytokine secretion, metabolism restriction and tumor cell proliferation inside the TME to influence cancer immunotherapy effects
Antibodies against PD-1/PD-L1
PD-1 is a coinhibitory transmembrane receptor expressed on tumor-infiltrating lymphocytes (TILs) [179]. Within the TME, PD-1 binds to PD-L1 and consequently inhibits CTL-mediated cytolysis, as well as Fas-induced cellular apoptosis, thus allowing tumor cells proliferate indefinitely [180, 181]. Inhibitors of PD-1/PD-L1, such as nivolumab, pembrolizumab, and atezolizumabor, promote immune responses against cancer cells in clinical trials [182,183,184,185,186,187].
Moreover, landmark experiments have confirmed the association between antibodies against PD-1/PD-L1 and the gut microbiota. These preclinical trials have explored the hallmark mechanisms of this crosstalk: (1) alterations in the gut microbiota composition caused by immune checkpoint inhibitors(ICIs), (2) the effects of gut microbes on intestinal immune cells, (3) induced metabolic changes affecting the immune response of commensals, and (4) the accumulation of immunocytes in the TME caused by the gut microbiota. Specifically, this crosstalk was first explored by Sivan et al. [22]. Their data suggested that Bifidobacterium could augment DC functions and enhance CD8 + T-cell priming and accumulation in the TME. Routy et al. [188] confirmed the correlation between the abundances of different microbes (A.muciniphila and E.hirae) and PD-1/PD-L1 blockade efficacy. Mechanistically, these researchers demonstrated that the antitumor effect was restored in an IL-12-dependent manner by increasing the recruitment of CCR9 + CXCR3 + CD4 + T lymphocytes into the TME. Another study indicated that Prevotella and A.muciniphila improved the therapeutic efficacy of PD-1/PD-L1 inhibitors and Bacteroides led to poorer efficacy. Researchers have speculated that changes in the gut microbiota affect glycerophospholipid metabolism, thereby altering the expression of IFN-γ and IL-2 in the TME [189]. In mice with breast cancer (BC), anti-PD-1 therapy increased the abundance of Bifidobacterium, Lactobacillus, and Adlercreutzia [190].
Analogous clinical studies were implemented in the following years, and the results validated the correlation between the gut microbiota composition and the therapeutic efficacy of ICIs in clinical trials beyond preclinical models.
In trials involving metastatic melanoma (MM) patients, contradictory results showed that no single species could be regarded as an entirely consistent predictive factor. In terms of mechanism, Gopalakrishnan et al. [191] reported increased abundances of Clostridiales, Ruminococcaceae, and Faecalibacterium in responders(R) and suggested that increasing antigen presentation and improving effector T-cell function in the TME could enhance antitumor immune responses. Matson et al. [192] performed FMT to transfer R-enriched bacteria into colonized mice and observed an increased frequency of DCs and augmented T-cell responses. Other studies have shown that specific bacterial species are associated with R and nonresponders(NRs) [193, 194] and that carriers of specific bacterial taxa exhibit a better cancer prognosis [195, 196].
Multiple studies on the systemic immune responses of cancer patients ranging from those with melanoma to those with non-small cell lung carcinoma (NSCLC) have detected a greater frequency of memory CD8 + T cells and NK cells in the periphery of R enriched with Alistipes putredinis Bifidobacterium longum, and Prevotella copri [197]. A group in the United States found that mice model with transplanted gut microbes had improved ICI efficacy when the TME was enriched with immunocytes [198]. Other studies have also demonstrated a diverse array of molecular features in the gut microbiota during immunotherapy modulation [199,200,201,202,203,204,205,206]. Taken together, the findings are conflicting; thus, continued research efforts are needed to establish causal relationship between different microbes and ICI treatment efficacy. Similarly, studies focusing on other rare thoracic malignancies are needed, although initial data have been provided [207].
Not until 2019 did studies start focusing on predicting responses to PD-1/PD-L1 immunotherapy based on the gut microbiota composition in the context of hepatocellular carcinoma (HCC). Zheng et al. [208] reported that the dynamic nature of commensals plays an important role in ameliorating oxidative stress injury and host inflammatory responses in antitumor therapy. Another study revealed that the antitumor functions of certain bacterial species could be a result of SCFA production and bile acid metabolism [209]. Although multiple studies have demonstrated that better ICI efficacy in HCC patients appears to be correlated with a favorable gut microbiota [210,211,212], one recent study failed to confirm such a positive association in patients with HCC [213].
Compared with those of the solid tumors mentioned above, little is known about the direct impact of individual intestinal nonpathogenic bacteria on the therapeutic outcomes of ICIs in renal cell carcinoma (RCC). Derosa et al. [214] observed a positive association between D. formicigenerans and CD8 + CD69 + T cells as well as negative associations between C. clostridioforme and CD137/4.1BB expressing CD4 + T lymphocytes and memory CXCR5-CCR6-CCR4-CCR10-CXCR3 + CD8 + T cells. Salgia et al. [215] also identified several species that were presumably correlated with therapeutic benefits.
Although a significant amount of research has been dedicated to revealing how the gut microbiota influences the carcinogenesis of colorectal carcinoma (CRC), little is known about the regulatory mechanisms involved in the efficacy of ICIs. In a recent study, F. nucleatum was connected to the activation of the stimulator of interferon genes (STING) signaling pathway as well as the accumulation of IFN-γ + CD8 + TILs [216]. To better understand how individual bacterial species modulate ICI therapy, future studies are needed to better characterize any shared functionalities among different microbial communities.
The negative impact of H. pylori on immunomodulation raises the concern that H. pylori infection may suppress immune responses to cancer immunotherapy [217, 218]. Researchers have confirmed that H. pylori infection decreases the effectiveness of cancer immunotherapies by inhibiting DCs and suppressing CD8 + T-cell responses [219].
Antibodies against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)
CTLA-4 is a major negative receptor of T cells and has upregulated expression upon T-cell activation [220,221,222,223,224,225,226]. Inhibitors of CTLA-4, such as ipilimumab and tremelimumab, are thought to boost antitumor immunity due to the strong immunosuppressive effects of CTLA-4 [227,228,229,230,231]. Mechanistically, anti-CTLA-4 blockade affects the Th1 subset of CD4 T cells that express an inducible costimulator (ICOS) [232, 233]. Additionally, both effector T cells and Tregs are the primary targets of anti-CTLA-4 mediated blockade [234, 235].
Studies have revealed the mechanisms by which different species of gut microbiota improve the clinical outcomes of anti-CTLA-4 immunotherapy. Initially, an altered gut microbiota was thought to activate IL-12-dependent Th1 immune responses, thereby facilitating antitumor effects [236, 237]. Chaput et al. [238] confirmed that prolonged progression-free survival (PFS) and overall survival (OS) in patients enriched with Firmicutes was mediated by increased ICOS induction levels of CD4 + T cells and sCD25 levels. A recent study suggested that the antitumor efficacy of CTLA-4 blockade is negatively correlated with the proportion of the microbial metabolite butyrate since systemic butyrate is capable of inhibiting ipilimumab-mediated DC maturation and the CD28 signaling pathway (Additional file 1: Table S1) [239].
Adoptive cell transfer (ACT)
While ICI efficacy relies on the presence of tumor-reactive T cells [240], ACT may be a good strategy for treating poorly immunogenic types of cancer [241]. There are two approaches to ACT: (1) isolating TILs from the TME and (2) genetically modifying blood-derived T cells to express chimeric antigen receptor (CAR). Both approaches require in vitro T-cell manipulation before reinfusion into patients [242,243,244,245,246,247]. Considering the obstacles to the application of ACT, interventions modulating the immune microenvironment, such as gut microbiota modifications, have become a central issue [248, 249].
Paulos et al. [250] reported for the first time that translocated microbes could augment the function of ACT therapy by triggering the TLR4 pathway. Activating this pathway stimulates DCs and increases the secretion of proinflammatory cytokines in the gut. Similarly, other studies also revealed enhanced ACT efficacy after vancomycin supplementation, which induced IL-12 expression to increase the number and activity of tumor-specific TILs [251]. Adoptive transfer of naïve Helicobacter hepaticus (Hhep)-specific CD4 + T cells has been shown to contribute to antitumor immunity in CRC. Mechanistically, researchers have discovered that increased Hhep levels stimulate tertiary lymphoid structures (TLSs), which further activate NK cells and CD4 + T cells [252]. Recently, Smith et al. [253] demonstrated a close correlation between a high abundance of Ruminococcus, Bacteroides, and Faecalibacterium and better responses to CD19 CAR T-cell therapy in patients. Collectively, these findings, although preliminary, have not revealed the exact mechanisms by which bacterial taxa and metabolites influence ACT immunotherapy outcomes, especially CAR-T-cell therapy outcomes (Additional file 1: Table S1) [254].
Unmethylated cytidine phosphate guanosine oligonucleotide (CpG-ODN) therapy
CpG-ODNs possess immunostimulatory effects and potential antitumor activity [255]. They interact with TLR9 in B cells and plasmacytoid DCs to initiate a signaling cascade that activates the NF-κB pathway and various cell types and induces the production of cytokines and chemokines [256]. Thus, CpG-ODN injections were initially promoted for their immunotherapeutic potential, and recent studies have focused on applying CpG-ODNs as an adjuvant to other cancer treatments [257,258,259].
Iida et al. [119] identified several species associated with CpG-ODN efficacy. These associations suggest that the gut microbiota affects immunotherapy by inducing TNF production and manipulating tumor-associated myeloid cells. These findings confirmed that commensals affect the outcomes of patients receiving CpG-ODN therapy by regulating inflammatory responses in the TME (Additional file 1: Table S1).
Microbial metabolites and the efficacy of cancer immunotherapy
Metabolites derived from the gut microbiota have been identified as important regulators of the development and function of immune cells [17, 260, 261]. Given their complicated interactions with the immune system, multiple studies have focused on how they impact local and systemic antitumor immune responses, especially in the context of ICI therapy (Fig. 4). These heavily studied metabolites can be divided into three subgroups according to their origin and synthesis: (1) metabolites produced by the gut microbiota from dietary components, (2) metabolites produced by the host and modified by the gut microbiota, and (3) metabolites synthesized de novo by the gut microbiota. We will discuss the latest evidence about the potential mechanisms underlying these interactions for each of these groups.
Metabolites produced by the gut microbiota from dietary components
SCFAs
In the intestine, dietary fiber can be fermented into SCFAs by the gut microbiota [262]. These SCFAs act as signaling molecules that regulate host physiology and immune processes, specifically by inhibiting HDACs or activating G protein-coupled receptors (GPRs) [87, 263,264,265,266]. Multiple studies have confirmed the association between gut microbiota-derived SCFAs and the long-term benefits of ICI treatment in cancer [202, 267,268,269]. However, Coutzac et al. [239] identified the antagonist effect of SCFAs that limits anti-CTLA-4 activity. Here, we will discuss the critical role that SCFAs play in the immune system, which demonstrates their antitumor effects in cancer immunotherapy.
SCFAs directly inhibit the proliferation of tumor cells. Researchers have shown that butyrate can inhibit tumor cell proliferation by decreasing the activation of nuclear factor of activated T-cell (NFAT)c3 and calcineurin [267]. Additionally, propionate produced by A. muciniphila promotes tumor cell apoptosis [268] In addition, SCFAs can induce histone hyperacetylation by inhibiting HDACs, leading to cell cycle arrest [269].
Moreover, SCFAs activate immune cells to augment antitumor immune responses. SCFAs can modulate intestinal macrophages and DCs through the inhibition of HDACs [87, 265, 270, 271]. Research has also shown that SCFAs modulate the suppressive function and differentiation of Foxp3 + Treg cells in an HDAC-dependent manner to establish immunological homeostasis in the gut [175, 177, 178, 272]. Singh et al. [273] showed that the GPR-butyrate interaction is another signaling factor that is involved in the differentiation of Treg cells. SCFAs also improved the efficacy of anticancer therapy by influencing cytotoxic CD8 + T cells. The antitumor effect was boosted by the inhibition of class I HDAC enzymes via an IL-12-dependent signaling pathway [274, 275]. The metabolic promotion of glycolysis and oxidative phosphorylation in CD8 + T cells induced by SCFAs provides energy for immune cells [276]. In addition, SCFAs increase acetyl-CoA levels to modulate energy metabolism in B cells to support antibody production [112].
There are also contradictory findings showing restricted antitumor activity of anti-CTLA-4 in the face of high systemic levels of butyrate [239], leading to poor clinical response to treatment with ICIs. Although the mechanism through which SCFAs affect the efficacy of ICIs remains ambiguous, the SCFA-associated immunomodulatory pathway and its relevant clinical trials are still a promising area of research.
Tryptophan catabolites
Tryptophan catabolites, which mostly result from the degradation of dietary proteins, are critical contributors to intestinal and systemic homeostasis [277]. These proteins act as ligands for the aryl hydrocarbon receptor (AhR) [278], which is a ligand-inducible transcription factor in host cells that assists in immune responses [279, 280]. Accumulating evidence has confirmed the antitumor effect of targeting these microbial metabolites in cancer treatment.
Clinical research has shown that a decreased ratio of serum kynurenine(Kyn)/ Trp improves ICI treatment efficacy [281, 282]. In concert, studies have further demonstrated that T-cell proliferation can be inhibited by high Kyn/Trp ratios, which consequently worsens patient prognosis [283]. Another clinical trial revealed the immunosuppressive activity of 3-hydroxyanthranilic acid (3-HAA), which is a downstream metabolite in the kynurenine pathway [284].
High levels of AhR expression have been recognized as a signal for rapid disease progression. Hezaveh et al. [285] observed the activation of AhR in tumor-associated macrophages (TAMs) by microbiota-derived tryptophan metabolites in pancreatic ductal adenocarcinoma (PDAC). Moreover, deletion of AhR reduced tumor growth, increased the number of IFNg + CD8 + T-cells, and improved the efficacy of ICI treatment.
Indole-3-carboxaldehyde (3-IAld) exhibits great potential in modulating the immune response at the interface between microbes and the host immune system [286]. Researchers have found that 3-IAld in alters the composition of the gut microbiota and induces SCFAs production [287]. In addition, 3-IAld has been shown to alleviate irAEs by activating the AhR/IL-22 pathway, which targets the epithelial barrier to help maintain mucosal homeostasis [288].
According to Huang et al. [289], interventions such as prebiotics assist in the accumulation of the tryptophan catabolite valeric acid. Decreased Kyn/Trp ratios could suppress Treg cells and activate effector T cells, which will eventually enhance the efficacy of anti-PD-1 immunotherapy. In summary, these findings support the oncogenic effect of the kynurenine pathway and the antitumor effect of indoles.
Metabolites produced by the host and modified by the gut microbiota
Bile acids
Bile acids (BAs) are a group of metabolites synthesized from cholesterol and then formed by the gut microbiota [290]. Limited knowledge is available regarding the correlation between ICI treatment outcomes and BAs, while relatively more is known about the mechanism through which BAs modulate the host immune system.
A recent study revealed distinct BA features in Rs and NRs to ICI-treated HCC. Specifically, ursodeoxycholic acid (UDCA) was significantly more abundant in Rs, whereas lithocholic acid (LCA) was more abundant in NRs [291]. The antitumor effect of UDCA has been widely reported [292]. Various signaling pathways, immune cells, and cytokines, such as the epidermal growth factor receptor (EGFR)/ERK signaling pathway, NKT cells, and TGF-β, are involved in the protective effect of UDCA [293,294,295].
Secondary BAs such as deoxycholic acid (DCA) activate EGFR and protein kinase C, thus causing DNA damage and apoptosis and eventually leading to cancer cell proliferation [296,297,298,299].
Metabolites synthesized de novo by the gut microbiota
Inosine
A recent study identified that A. muciniphila and B. pseudolongum utilize the inosine-adenosine 2A receptor(A2AR) signaling pathway to improve the efficacy of ICI therapy. The authors presumed that inosine activates T cells and reprograms the TME [300]. Based on their findings and other relevant studies, we identified several potential mechanisms through which inosine may influence immune responses to ICI therapy.
The immunomodulatory effects of inosine on immune cells could be a critical factor. Activation of the inosine-A2AR-cAMP-PKA signaling pathway leads to phosphorylation of the transcription factor cAMP response element–binding protein (CREB) [300]. Other research has shown that the microbiota–inosine–A2AR axis can influence the differentiation and expansion of Treg, CD8 + T, Th1, and Th2 cells and the production of cytokines [301,302,303,304,305].
Furthermore, inosine can support cell growth and T-cell functions as an alternative metabolic substrate. The high metabolic demands of cancer cells can limit the capacity of effector T cells by restricting available nutrients [306,307,308]. Wang et al. [309] demonstrated that inosine can relieve tumor-imposed metabolic restrictions on T cells. Specifically, effector T cells utilize the ribose subunit of inosine to activate central metabolic pathways and generate ATP and biosynthetic precursors.
Peptidoglycan
In a recent study, NOD2-active muropeptides generated by active enterococci with orthologs of the NlpC/p60 peptidoglycan hydrolase SagA were shown to improve the efficacy of ICI immunotherapy [310]. Further mechanistic exploration revealed that microbiota-derived peptidoglycans augment CD8 + T cells that express granzyme B and a particular monocyte population characterized by Cx3cr1 and Nr4a1 expression [39]. Accordingly, researchers suggested that specialized peptidoglycan remodeling activity and muropeptide-based strategies could be regarded as the future of next-generation immunotherapy.
Immune-related adverse events and the gut microbiota
A large spectrum of autoimmune responses is associated with ICIs due to their impact on immune cell activation [311]. Inflammatory side effects termed immune-related adverse events (irAEs) are frequently linked to the gastrointestinal tract, endocrine glands, skin, and liver during ICI treatment [312,313,314,315,316]. These potential irAEs reveal the necessity of multidisciplinary, collaborative management across the clinical spectrum [317, 318]. In addition to identifying microbial signatures associated with the efficacy of ICI therapy, the microbiota composition and dysbiosis in the gut have also shown a connection with the incidence of irAEs (Additional file 1: Table S2).
In terms of immunotherapy-related colitis, multiple studies have identified various microbial signatures and related signaling pathways that mediate the proinflammatory side effects of ICIs. Dubin et al. [319] reported a correlation between the abundance of specific bacterial taxa and subsequent colitis development. This report was followed by several studies that identified more irAE-colitis-associated gut microbes ranging from Firmicutes families to Streptococcus spp [196, 200, 209, 236, 238]. In addition to studies on colitis-induced bacteria, other studies have suggested that Bifidobacterium ameliorates colitis [320]. Researchers have demonstrated that Bifidobacterium breve and Lactobacillus rhamnosum can enhance the suppressive function of Treg cells by stimulating an IL-10/IL10Ra signaling loop [321].
These discoveries have provided opportunities to target gut microbes using strategies such as FMT or probiotics to decrease intestinal toxicity. Researchers in a case series utilizing FMT to abrogate ICI-associated colitis observed an increase in the proportion of Treg cells within the colonic mucosa [322]. Additionally, administration of the probiotic L. reuteri could ameliorate the immunopathology associated with ICIs by affecting the local number of ILC3s [323]. The microbial metabolite 3-IAld has demonstrated therapeutic potential in maintaining epithelial barrier function in the gut, which could help alleviate ICI-induced intestinal toxicity [286].
With the increased use of ICIs, irAEs are no longer limited to colitis but include all kinds of related diseases, such as diarrhea, pancreatitis, pruritus, and thyroid dysfunction. Researchers have identified various characteristics of the gut microbiome related to the increasing risk of irAEs [324,325,326]. Usyk et al. [327] applied this widely studied connection to predict the incidence of irAEs.
In summary, utilizing the microbiota composition as a prediction tool and therapeutic target for irAEs in ICI-treated patients may be a promising direction for treatment.
Gut microbiota modifications in response to cancer immunotherapy
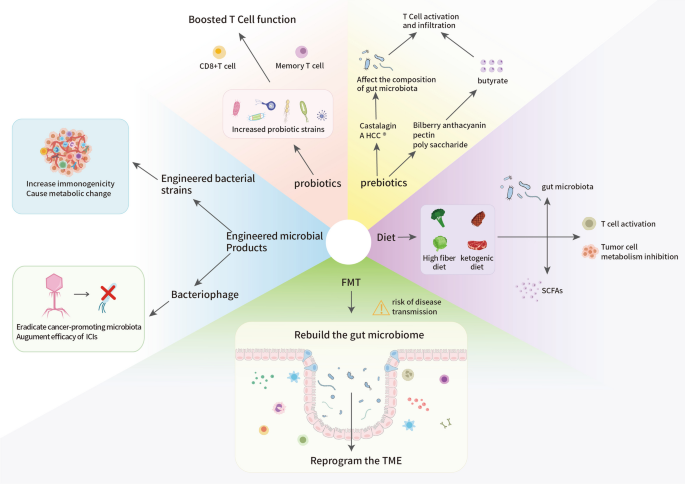
Accumulating evidence has revealed how the gut microbiota and its metabolites interact with the host immune system to regulate antitumor immunity and immunotherapy responses. Therefore, modifications of the gut microbiota to enhance ICI treatment efficacy are promising approaches for therapeutic development. Here, we review preclinical and clinical trials that aimed to improve the clinical outcomes of patients treated with ICIs by altering gut microbes (Fig. 5). The main methods used for this purpose include FMT, dietary regulation, probiotics, prebiotics, and engineered microbial products.
Future intervention strategies to modificate gut microbiota in cancer immunotherapy. Targeting the association between the gut microbiome and cancer immunotherapy, modifying the gut microbiota with the latest intervention technologies could significantly advance the quality of individualized treatment. Listed here are the potential mechanisms behind the five microbiota modification strategies, which could be used to promote the efficacy of cancer immunotherapy in a precise manner. These intervention strategies are developed mainly based on current views of the crosstalk between the gut microbiota and the immune system. FMT, dietary regulation, probiotics, prebiotics, and engineered microbial products all can alter intestinal bacteria to enhance anti-tumor immune responses inside the TME, which consequently improve the efficacy of cancer immunotherapy
FMT
FMT is a well-established clinical approach for modulation of the gut microbiota [328]. Transplantation of the gut microbiota from a healthy donor restores intestinal microbial diversity in the recipient [329]. Currently, FMT is recommended by the FDA for treating recurrent Clostridium difficile infection [330].
Considering the unique microbial features of ICI responders, it is tempting to presume that FMT is applicable in immunotherapy. Several preliminary trials have explored coupling FMT with immunotherapy, and their results have indicated that FMT could induce the differential expression of T-cell and NK cell-related pathways in ways that control tumor growth and ameliorate the immune response [188, 191, 192, 331].
Three recent studies have investigated the feasibility of introducing FMT through oral stool capsules in patients treated with ICIs. All of these studies revealed desirable outcomes, including an increased abundance of bacteria associated with response to anti-PD-1 therapy, activation of CD8 + T cells, and a decreased amount of IL-8-expressing myeloid cells. The microbiota sources were obtained from healthy stool donors [23, 261, 332]. These observations confirmed that FMT could alter the microbiota composition and reprogram immune and inflammatory factors to increase the efficacy of ICIs [333]. The safety data from Routy et al. [332] confirmed that FMT combined with anti-PD-1 therapy did not increase the incidence of irAEs. Additionally, Spreafico et al. utilized a microbial consortium, Microbial Ecosystem Therapeutic 4 (MET4), as an alternative to FMT in combination with ICIs in patients with advanced solid tumors. Their results suggested no worsening of ICI-associated irAEs when using MET4 [334]. Given these promising results, there are many ongoing clinical trials investigating the exact mechanism behind FMT-induced enhancement of ICI efficacy in larger patient cohorts (Additional file 1: Table S3).
Recently, two live microbiome therapeutic products were approved by the FDA: RBX2660 and SER-109. Clinical trials on these products have shown that they reduce the incidence of recurrent Clostridioides difficile infection (rCDI) with a low risk of adverse events related to treatment. We summarized the detailed trial design and results of these products(Table 3).
Based on their innovativeness, RBX2660 and SER-109 were granted Breakthrough Therapy Status, Fast Track, and Orphan Drug designations by the FDA [335, 336].
However, there is also considerable risk during FMT [337]. For example, a whole transplantation of the gut microbiota may sabotage the existing boundary of beneficial bacteria in the recipient, thereby causing infectious diseases [338]. Therefore, professional guidelines should be put in place to mandate presurgical safety screenings for donors, define standardized duration and delivery methods for the procedure, and build machine learning models that can to predict responses to minimize FMT-associated risks [339,340,341].
Dietary regulation
Recent studies have revealed the potential regulatory effect of diet on the gut microbiota [342]. Multiple studies have proven that dietary interventions can alter the composition of the gut microbiome. For instance, the standard Western diet (which is high in fat and carbohydrates and low in fiber) could induce gut dysbiosis, as it causes an increase in Firmicutes, Proteobacteria, Mollicutes, Bacteroides spp., Alistipes spp., Bilophila spp., Enterobacteriaceae, Escherichia, Klebsiella, and Shigella while decreasing the abundance of beneficial bacteria Bacteroidetes, Prevotella, Lactobacillus spp., Roseburia spp., E. rectale, Bacillus bifidus and Enterococcus, leading to increased BA secretion and decreased downstream SCFA production [343,344,345]. Moreover, low-fat, high-fiber diets can improve the gut microbiome composition by shifting the microbiota composition toward and increase in the beneficial bacteria Prevotella and Bacteroides and a decreased in Firmicutes [346]. Therefore, dietary regulation via the gut microbiota could be a promising clinical strategy to improve the efficacy of cancer treatment [347,348,349,350,351,352].
One clinical study that focused on the impact of the food-gut axis on the response to ICIs revealed a positive correlation between high-fiber diets and improved responsiveness to anticancer immunotherapy. Specifically, higher expression of genes related to T-cell activation and the interferon response were observed in the high-fiber diet group, which were likely induced by fiber-fermenting bacteria through the production of SCFAs [353].
A ketogenic diet, which is a high-fat, low-protein, and low-carbohydrate diet, is well known for its ability to inhibit lactate-mediated tumoral immunosuppression and tumor cell metabolism [354,355,356]. Ferrere et al. studied the efficacy of combining a ketone-rich diet with immunotherapy [357] and reported that supplementation with ketone bodies could re-establish therapeutic responses when ICI treatment failed to reduce tumor growth on its own. A ketogenic diet could induce changes in the gut microbiota composition, leading to the expansion of CXCR3 + T cells and inhibition of the IFNγ-mediated upregulation of PD-L1 expression on myeloid cells.
Currently, many tentative clinical trials aimed at characterizing diet-induced alterations in the gut microbiota and their possible effects on immunotherapy efficacy are underway to better understand their relationship (Additional file 1: Table S3).
Probiotics
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” [358]. Probiotics have been applied to prevent and treat multiple diseases [355,356,357] and specifically for cancer, Lactobacillus spp. and Bifidobacterium spp. strains were capable of relieving dysbiosis, enhancing anticancer immunity, and improving ICI treatment efficacy in recent studies [359,360,361,362].
The utilization of single probiotic strains has yielded exciting therapeutic effects when combined with cancer immunotherapy. Bifidobacterium supplementation has been shown to play a key role in improving ICI efficacy [22, 363]. The probiotics Clostridium butyricum and Lactobacillus rhamnosus, and antibiotic-resistant lactic acid bacteria may also improve the therapeutic efficacy of ICIs as they increase the number of beneficial bacteria and reshape functional metagenomes [24, 364,365,366]. In terms of A. muciniphila, researchers have identified an IL-12-dependent mechanism by which A. muciniphila triggers the recruitment of CCR9 + CXCR3 + CD4 + T lymphocytes into the TME to increase the efficacy of ICI treatments [188]. Increased T-cell function was also observed in CTLA-4 mAb-treated patients administered L.acidophilus. Zhuo et al. [367] reported that ICI efficacy could be enhanced by increasing the abundance of CD8 + T cells and effector memory T cells, as well as by decreasing the abundance of Treg cells and M2 macrophages in the TME.
Compared to single probiotic strains, a bacterial consortium may better represent the collective properties of the gut microbiota. Tanoue et al. [368] applied a bacterial consortium containing 11 commensal strains in tumor-bearing mice and identified a mechanism or enhancing ICI efficacy that was dependent on CD103 + DCs and major histocompatibility class Ia cells. A recent study validated the use of probiotics as a stand-alone therapy for treating tumors, where a mix of four Clostridiales species could exert antitumor effects by activating CD8 + T cells and increasing the immunogenicity of tumors [369].
Nevertheless, there is conflicting evidence on the benefits of probiotics marketed as dietary supplements [370]. Suez et al. [371] identified a delayed reconstitution of the gut mucosal microbiota using an 11-strain probiotic cocktail. Inconsistent clinical results also exist of the agonist effects of probiotic strains and formulations in immunotherapy have also been reported [353]. More efforts are needed to gain a thorough understanding of the effects of probiotics on immune responses and cancer immunotherapy (Additional file 1: Table S3).
Prebiotics
A prebiotic is defined as a substrate that is selectively utilized by host microorganisms to confer a health benefit [372]. Studies have shown that prebiotics can assist in promoting immunomodulatory effects, as well as stimulating the gut barrier and enhancing metabolic functions [373].
Prebiotics may improve the immunomodulatory effects of ICIs by altering the adundance of SCFAs. Researchers have shown that natural prebiotics, such as bilberry anthocyanin, pectin, the plant polysaccharide inulin, and ginseng polysaccharides, modulate anti-PD-1 therapy. These prebiotics can increase the amount of beneficial SCFAs, which further induces systemic memory T-cell responses and increases T-cell infiltration and activation in the TME [289, 374,375,376,377]. Alternatively, artificial prebiotics such as AHCC® (a standardized extract of cultured Lentinula edodes mycelia) and castalagin also enhanced ICI efficacy by altering the gut microbiota composition and enhancing T-cell functions within the TME [378, 379].
Engineered microbial products
With the development of genetic technology, engineered microbial products have attracted research interest worldwide. In contrast to the innate microbiota, these engineered microbes are designed to be sensitive to disease signals and respond to them at the site of onset [380]. They also contain bacteriophages, which modulate the composition of the gut microbiota.
To date, multiple reports have demonstrated the reliable delivery of antitumor benefits by engineered bacterial strains in many different contexts [381,382,383,384,385]. Here, we discuss how these microbes could be applied as a complement to anticancer immunotherapy. Binder et al. [386] demonstrated a powerful new therapeutic approach, that combines Salmonella typhimurium with PD-L1 blockade to activate the expansion of tumor-specific CD8 + T cells, resulting in the eradication of tumors. Similarly, Mkrtichyan et al. [387] observed an increase in CD8 + T-cell infiltration and antigen-specific immune responses in the periphery during anti-PD-1 immunotherapy after the administration of Listeria monocytogenes. These studies supported the hypothesis that microbes could indeed establish a more immunogenic microenvironment. Another approach to improve antitumor effects would be to enable metabolic modulation. Intertumoral injection of the Nissle 1917 E.coli strain increased the intracellular L-arginine concentration, triggered T-cell infiltration, and amplified the efficacy of PD-L1 blockade [388]. However, further technical refinements are still needed before the full-fledged clinical application of engineered bacteria can be achieved [389].
The utilization of bacteriophages as microbe-targeting vectors to induce immunomodulation has attracted extensive research interest [290, 390]. Bacteriophages promote the eradication of cancer-promoting commensals while maintaining their influence on the surrounding microbiota. A bacteriophage-guided, biotic–abiotic hybrid nanosystem could also provide precise phage release within the TME to accurately remove only pro-tumoral bacteria. For instance, F. nucleatum-specific phages have been shown to augment the efficacy of ICIs as well as first-line chemotherapy treatments [391, 392]. Notably, studies have revealed that correlations between specific bacteriophages and bacteria appear to be associated with FMT outcomes [393, 394].
These engineered microbial products are promising for immunotherapy development, and more studies are needed to explore their potential application.
Challenges and future perspectives
In this review, we systematically examined current studies on the intricate relationship between the gut microbiota and the host immune system. Given the dynamic interactions among the gut microbiota, its metabolites, and various cancer immunotherapies including ICI, ACT, and CpG-ODN therapy, future studies should focus on discovering the underlying mechanisms of this modulatory effect, in addition to investigating distinct microbiota compositions. Recently, there has been accumulating evidence that the gut microbiota is a leading cause of irAEs in cancer immunotherapy. To minimize irAEs and improve immunotherapy safety, more studies are needed to develop novel interventions targeting commensal bacteria. Additionally, after reviewing the current therapeutic trials utilizing FMT, diet control, probiotics, prebiotics, and engineered microbial products combined with immunotherapy, we believe that there is still a tremendous need to explore the design of personalized methods of microbiota modification and strategies to optimize therapeutic efficacy.
Recent research on microbiota-cancer immunotherapy interactions shares the common concern of heterogeneity in trial design [5], which can be attributed to the lack of a uniform methodology during sample allocation, technology utilization, data quality control, and data analysis. To address this issue, a consortium-level effort is needed to construct a standardized protocol specifying certain requirements for microbial specimen type and origin, sample handling environment, and microbiota bioinformatics analysis [395]. In addition to the study design, dynamic alterations in the gut microbiota and time-dependent disease progression could also induce heterogeneity [396, 397]. Therefore, consistent monitoring of the microbial composition throughout the disease course or exploration of the predictable patterns of microbial communities needs to be incorporated as a part of study protocols [398]. A recent study developed a computational method that exhibited promising potential for monitoring the dynamic alterations in gut microbes. This approach revealed the associations between drug exposure and the microbiome at high resolution, indicating the capacity to predict microbial changes and patient outcomes [399].
Moreover, the high degrees of biological inter- and intrapersonal variability of the gut microbiota imply that there is much more to learn in terms of individual heterogeneity [400]. Emerging spatial multiomics tools, especially single-cell techniques, are invaluable in deciphering the heterogeneous configurations of individuals at the bacterial strain level [401, 402]. Despite the accumulating evidence of improved therapeutic outcomes in humans and preclinical model mice, there are still gaps in our knowledge regarding the modulating effects of the gut microbiota that hindering its clinical application. Most importantly, most studies have focused solely on observing the correlation between the gut microbiota and treatment outcomes rather than exploring the existence of any causality. Because the gut microbiota functions as a whole, the impact of modifying individual bacterial strains may have different effects on the collective properties of the entire gut microbiota beyond an individual strain. To advance the current research from association-based to mechanism-based, the application of synthetic biology in the human microbiota might be a critical tool [403, 404].
In terms of gut microbiota modification, more functional studies and prospective clinical trials are needed to translate preclinical interventions targeting the gut microbiota into clinical applications in humans. One main challenge of applying experimental interventions in the clinic is that humans and animals do not share the same immune system. Another factor that cannot be ignored is differences in the gut microbiome composition and richness between rodents and humans. These limitations have restricted the translation of preclinical studies focusing on the gut microbiota. Therefore, the construction and characterization of the human gut microbiota in vitro could significantly improve the quality of individualized immunotherapy [405]. Furthermore, in situ genome engineering of the microbiota has also demonstrated promising potential for the regulation of existing microbial communities, which suggests its future utilization in the manipulation of cancer immunotherapy outcomes [406].
In summary, our knowledge about the intricate relationships among the gut microbiota, the host immune system, and cancer immunotherapy are still limited. By combining artificial intelligence applications with the emerging advances we mentioned above [407], future research should provide further insights into the crosstalk between the microbiota and clinical outcomes of immunotherapies, thus paving the way for the clinical application of gut microbiota interventions, as well as the development of personalized medicine for cancer management.
Availability of data and materials
Not applicable.
Abbreviations
- SCFAs:
-
Short-chain fatty acids
- TMAO:
-
Trimethylamine N-oxide
- GF:
-
Germ-free
- PD-1/PD-L1:
-
Programmed cell death protein-1/programmed cell death protein-1-ligand 1
- FMT:
-
Fecal microbiota transplantation
- GI:
-
Gastrointestinal
- CTX:
-
Cyclophosphamide
- Th cell:
-
Helper T cell
- Treg cell:
-
Regulatory T cell
- AMPs:
-
Antimicrobial peptides
- HD:
-
Human defensing
- PRRs:
-
Pattern recognition receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- IECs:
-
Intestinal epithelial cells
- TLRs:
-
Toll-like receptors
- NOD:
-
Nucleotide oligomerization domain
- NLRs:
-
Nucleotide-binding domain and leucine-rich repeat-containing receptors
- PI3K:
-
Phosphoinositide 3-kinase
- MyD88:
-
Myeloid differentiation primary response gene 88
- ZO1:
-
Zona occludens 1
- MLCK:
-
Myosin light chain kinase
- PSA:
-
Polysaccharide A
- B. fragilis :
-
Bacteroides fragilis
- IL-10:
-
Interleukin-10
- ERK:
-
Extracellular-signal-regulated kinases
- MAPK:
-
Mitogen-activated protein kinases
- NLRP:
-
NOD-like receptor thermal protein domain associated protein
- P. mirabilis :
-
Proteus mirabilis
- A. muciniphila :
-
Akkermansia muciniphila
- TNF-α:
-
Tumor necrosis factor-α
- DCs:
-
Dendritic cells
- Csf2:
-
Colony-stimulating factor 2
- ILC3:
-
Innate lymphoid cells 3
- DCs:
-
Dendritic cells
- APCs:
-
Antigen-presenting cells
- pDCs:
-
Plasmacytoid dendritic cells
- cDCs:
-
Conventional dendritic cells
- IFN‐I:
-
Interferon-I
- OMVs:
-
Outer membrane vesicles
- NK cell:
-
Natural killer cell
- SFB :
-
Segmented filamentous bacteria
- BCR:
-
B cell receptors
- HDAC:
-
Histone deacetylase
- ATP:
-
Adenosine triphosphate
- Bregs:
-
Regulatory B cells
- CTLs:
-
Cytotoxic T lymphocytes
- TME:
-
Tumor microenvironment
- Tc17:
-
Cytotoxic T lymphocyte17
- Trp:
-
Tryptophan
- TGF:
-
Transforming growth factor
- Tfh cell:
-
Follicular helper T cell
- GC:
-
Germinal center
- Tfr cells:
-
T follicular regulatory cells
- ICIs:
-
Immune checkpoint inhibitors
- TILs:
-
Tumor-infiltrating lymphocytes
- BC:
-
Breast cancer
- MM:
-
Metastatic melanoma
- R:
-
Responders
- NR:
-
Non-responders
- NSCLC:
-
Non-small-cell lung carcinoma
- HCC:
-
Hepatocellular carcinoma
- RCC:
-
Renal cell carcinoma
- CRC:
-
Colorectal carcinoma
- STING:
-
Stimulator of interferon genes
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen-4
- ICOS:
-
Inducible costimulatory
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- ACT:
-
Adoptive cell transfer
- CAR:
-
Chimeric antigen receptor
- Hhep :
-
Helicobacter hepaticus
- TLSs:
-
Tertiary lymphoid structures
- CpG-ODN:
-
Unmethylated cytidine phosphate guanosine oligonucleotides
- GPRs:
-
G protein-coupled receptors
- NFAT:
-
Nuclear factor of activated T-cell
- AhR:
-
Aryl hydrocarbon receptor
- Kyn:
-
Kynurenine
- 3-HAA:
-
3-Hydroxyanthranilic acid
- TAM:
-
Tumor-associated macrophage
- PDAC:
-
Pancreatic ductal adenocarcinoma
- 3-IAld:
-
Indole-3-carboxaldehyde
- BAs:
-
Bile acids
- UDCA:
-
Ursodeoxycholic acid
- LCA:
-
Lithocholic acid
- EGFR:
-
Epidermal growth factor receptors
- DCA:
-
Deoxycholic acid
- A2AR:
-
Adenosine 2A receptor
- CREB:
-
CAMP response element–binding protein
- irAEs:
-
Immune-related adverse events
- rCDI:
-
Recurrent clostridioides difficile infection
References
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32.
The Integrative Human Microbiome Project. Nature 2019, 569(7758):641-648
Suzuki TA, Fitzstevens JL, Schmidt VT, Enav H, Huus KE, Mbong Ngwese M, Grießhammer A, Pfleiderer A, Adegbite BR, Zinsou JF, et al. Codiversification of gut microbiota with humans. Science. 2022;377(6612):1328–32.
Ting NL, Lau HC, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71(7):1412–25.
Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371(6536):eabc4552.
Zhao LY, Mei JX, Yu G, Lei L, Zhang WH, Liu K, Chen XL, Kolat D, Yang K, Hu JK. Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications. Signal Transduct Target Ther. 2023;8(1):201.
Goto Y, Iwata S, Miyahara M, Miyako E. Discovery of intratumoral oncolytic bacteria toward targeted anticancer theranostics. Adv Sci. 2023;10(20):e2301679.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30.
McNally L, Brown SP. Microbiome: ecology of stable gut communities. Nat Microbiol. 2016;1:15016.
Relman DA. The human microbiome: ecosystem resilience and health. Nutr Rev. 2012;70(Suppl 1):S2-9.
Shaw LP, Bassam H, Barnes CP, Walker AS, Klein N, Balloux F. Modelling microbiome recovery after antibiotics using a stability landscape framework. ISME J. 2019;13(7):1845–56.
Coyte KZ, Schluter J, Foster KR. The ecology of the microbiome: Networks, competition, and stability. Science. 2015;350(6261):663–6.
Claesson MJ, O’Sullivan O, Wang Q, Nikkila J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O’Toole PW. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE. 2009;4(8):e6669.
Verberkmoes NC, Russell AL, Shah M, Godzik A, Rosenquist M, Halfvarson J, Lefsrud MG, Apajalahti J, Tysk C, Hettich RL, et al. Shotgun metaproteomics of the human distal gut microbiota. ISME J. 2009;3(2):179–89.
Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70(3):595–605.
Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52.
Martin-Gallausiaux C, Marinelli L, Blottiere HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49.
Janeiro MH, Ramirez MJ, Milagro FI, Martinez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398.
Fernandes MR, Aggarwal P, Costa RGF, Cole AM, Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat Reviews Cancer. 2022;22:703–22.
Ignacio A, Shah K, Bernier-Latmani J, Köller Y, Coakley G, Moyat M, Hamelin R, Armand F, Wong NC, Ramay H, et al. Small intestinal resident eosinophils maintain gut homeostasis following microbial colonization. Immunity. 2022;55(7):1250-1267.e1212.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–9.
Tomita Y, Goto Y, Sakata S, Imamura K, Minemura A, Oka K, Hayashi A, Jodai T, Akaike K, Anai M, et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology. 2022;11(1):2081010.
Schupack DA, Mars RAT, Voelker DH, Abeykoon JP, Kashyap PC. The promise of the gut microbiome as part of individualized treatment strategies. Nat Rev Gastroenterol Hepatol. 2021;19:7–25.
Hitch TCA, Hall LJ, Walsh SK, Leventhal GE, Slack E, de Wouters T, Walter J, Clavel T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022;15:1095–113.
Zhang H, Zhang Z, Liao Y, Zhang W, Tang D. The complex link and disease between the gut microbiome and the immune system in infants. Front Cell Infect Microbiol. 2022;12:924119.
Spiljar M, Merkler D, Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, Mucosal Barrier, and SCFAs. Front Immunol. 2017;8:1353.
Brown EM, Kenny DJ, Xavier RJ. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu Rev Immunol. 2019;37:599–624.
Kiner E, Willie E, Vijaykumar B, Chowdhary K, Schmutz H, Chandler J, Schnell A, Thakore PI, LeGros G, Mostafavi S, et al. Gut CD4(+) T cell phenotypes are a continuum molded by microbes, not by T(H) archetypes. Nat Immunol. 2021;22(2):216–28.
Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science. 2017;357(6353):806–10.
Nowosad CR, Mesin L, Castro TBR, Wichmann C, Donaldson GP, Araki T, Schiepers A, Lockhart AAK, Bilate AM, Mucida D, et al. Tunable dynamics of B cell selection in gut germinal centres. Nature. 2020;588(7837):321–6.
Johansson ME, Jakobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, Arike L, Wising C, Svensson F, Bäckhed F, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18(5):582–92.
Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339–71.
Routy B, Letendre C, Enot D, Chénard-Poirier M, Mehraj V, Séguin NC, Guenda K, Gagnon K, Woerther PL, Ghez D, et al. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology. 2017;6(1):e1258506.
Staffas A, Burgos da Silva M, Slingerland AE, Lazrak A, Bare CJ, Holman CD, Docampo MD, Shono Y, Durham B, Pickard AJ, et al. Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe. 2018;23(4):447–57.
Guo H, Chou WC, Lai Y, Liang K, Tam JW, Brickey WJ, Chen L, Montgomery ND, Li X, Bohannon LM, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020;370(6516):eaay9097.
Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–81.
Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–31.
Zegarra-Ruiz DF, Kim DV, Norwood K, Kim M, Wu WH, Saldana-Morales FB, Hill AA, Majumdar S, Orozco S, Bell R, et al. Thymic development of gut-microbiota-specific T cells. Nature. 2021;594(7863):413–7.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6.
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM, Flament C, Lepage P, Roberti MP, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–43.
Bin Hafeez A, Jiang X, Bergen PJ, Zhu Y. Antimicrobial peptides: an update on classifications and databases. Int J Mol Sci. 2021;22(21):11691.
Ehmann D, Wendler J, Koeninger L, Larsen IS, Klag T, Berger J, Marette A, Schaller M, Stange EF, Malek NP, et al. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc Natl Acad Sci USA. 2019;116(9):3746–51.
Ahuja M, Schwartz DM, Tandon M, Son A, Zeng M, Swaim W, Eckhaus M, Hoffman V, Cui Y, Xiao B, et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab. 2017;25(3):635–46.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20.
Layunta E, Buey B, Mesonero JE, Latorre E. Crosstalk between intestinal serotonergic system and pattern recognition receptors on the microbiota-gut-brain axis. Front Endocrinol. 2021;12:748254.
Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, Barton GM. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial cell type-specific and temporal patterns. Immunity. 2018;49(3):560–75.
Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8(12):1327–36.
Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170(8):3977–85.
Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, Bulik-Sullivan E, Carroll IM, Hansen JJ, Chen L, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Investig. 2019;129(9):3702–16.
Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165(7):1708–20.
Spindler MP, Siu S, Mogno I, Li Z, Yang C, Mehandru S, Britton GJ, Faith JJ. Human gut microbiota stimulate defined innate immune responses that vary from phylum to strain. Cell Host Microbe. 2022;30(10):1481-1498.e1485.
Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, Gariboldi S, Zanobbio L, Arnaboldi F, Shirai YF, Serrao G, et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. 2007;178(7):4296–303.
Poling HM, Wu D, Brown N, Baker M, Hausfeld TA, Huynh N, Chaffron S, Dunn JCY, Hogan SP, Wells JM, et al. Mechanically induced development and maturation of human intestinal organoids in vivo. Nat Biomed Eng. 2018;2(6):429–42.
Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132(4):1359–74.
Nighot M, Al-Sadi R, Guo S, Rawat M, Nighot P, Watterson MD, Ma TY. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am J Pathol. 2017;187(12):2698–710.
Menendez A, Willing BP, Montero M, Wlodarska M, So CC, Bhinder G, Vallance BA, Finlay BB. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J Innate Immun. 2013;5(1):39–49.
Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7(4):818–28.
Alvarez LA, Kovacic L, Rodriguez J, Gosemann JH, Kubica M, Pircalabioru GG, Friedmacher F, Cean A, Ghise A, Sarandan MB, et al. NADPH oxidase-derived H2O2 subverts pathogen signaling by oxidative phosphotyrosine conversion to PB-DOPA. Proc Natl Acad Sci USA. 2016;113(37):10406–11.
Erturk-Hasdemir D, Oh SF, Okan NA, Stefanetti G, Gazzaniga FS, Seeberger PH, Plevy SE, Kasper DL. Symbionts exploit complex signaling to educate the immune system. Proc Natl Acad Sci USA. 2019;116(52):26157–66.
Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15(4):413–23.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20.
Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–10.
Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, Baines JF, Schreiber S, Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60(10):1354–62.
Trindade BC, Chen GY. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol Rev. 2020;297(1):139–61.
Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203(1):203–13.
Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41(2):311–24.
Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Núñez G. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34(5):769–80.
Macho Fernandez E, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60(8):1050–9.
Zhang XN, Yu ZL, Chen JY, Li XY, Wang ZP, Wu M, Liu LT. The crosstalk between NLRP3 inflammasome and gut microbiome in atherosclerosis. Pharmacol Res. 2022;181:106289.
Seo SU, Kamada N, Muñoz-Planillo R, Kim YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD, et al. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity. 2015;42(4):744–55.
Yao X, Zhang C, Xing Y, Xue G, Zhang Q, Pan F, Wu G, Hu Y, Guo Q, Lu A, et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat Commun. 2017;8(1):1896.
Chen GY, Liu M, Wang F, Bertin J, Núñez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186(12):7187–94.
Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, Baxter NT, Zhao L, Schloss PD, Martens EC, et al. NLRP6 Protects Il10(-/-) mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 2017;19(4):733–45.
Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–59.
Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163(6):1428–43.
Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178(3):1256–60.
Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20(5):649–60.
Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity. 2012;36(5):742–54.
Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37(1):96–107.
Chen L, Wilson JE, Koenigsknecht MJ, Chou WC, Montgomery SA, Truax AD, Brickey WJ, Packey CD, Maharshak N, Matsushima GK, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol. 2017;18(5):541–51.
Truax AD, Chen L, Tam JW, Cheng N, Guo H, Koblansky AA, Chou WC, Wilson JE, Brickey WJ, Petrucelli A, et al. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe. 2018;24(3):364-378.e366.
Delfini M, Stakenborg N, Viola MF, Boeckxstaens G. Macrophages in the gut: masters in multitasking. Immunity. 2022;55(9):1530–48.
Deng H, Li Z, Tan Y, Guo Z, Liu Y, Wang Y, Yuan Y, Yang R, Bi Y, Bai Y, et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci Rep. 2016;6:29401.
Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343(6178):1249288.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111(6):2247–52.
Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, Chomka A, Ilott NE, Johnston DGW, Pires E, et al. The Short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432-445.e437.
Wu K, Yuan Y, Yu H, Dai X, Wang S, Sun Z, Wang F, Fei H, Lin Q, Jiang H, et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood. 2020;136(4):501–15.
Galati D, Zanotta S. Dendritic cell and cancer therapy. Int J Mol Sci. 2023;24(4):e257–67.
Probst HC, Muth S, Schild H. Regulation of the tolerogenic function of steady-state DCs. Eur J Immunol. 2014;44(4):927–33.
Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity. 2019;50(1):37–50.
Schaupp L, Muth S, Rogell L, Kofoed-Branzk M, Melchior F, Lienenklaus S, Ganal-Vonarburg SC, Klein M, Guendel F, Hain T, et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell. 2020;181(5):1080-1096.e1019.
Durant L, Stentz R, Noble A, Brooks J, Gicheva N, Reddi D, O’Connor MJ, Hoyles L, McCartney AL, Man R, et al. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome. 2020;8(1):88.
Bessman NJ, Mathieu JRR, Renassia C, Zhou L, Fung TC, Fernandez KC, Austin C, Moeller JB, Zumerle S, Louis S, et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science. 2020;368(6487):186–9.
Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120.
Poggi A, Benelli R, Venè R, Costa D, Ferrari N, Tosetti F, Zocchi MR. Human gut-associated natural killer cells in health and disease. Front Immunol. 2019;10:961.
Ogawa T, Asai Y, Tamai R, Makimura Y, Sakamoto H, Hashikawa S, Yasuda K. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin Exp Immunol. 2006;143(1):103–9.
Sierra S, Lara-Villoslada F, Sempere L, Olivares M, Boza J, Xaus J. Intestinal and immunological effects of daily oral administration of Lactobacillus salivarius CECT5713 to healthy adults. Anaerobe. 2010;16(3):195–200.
Kosaka A, Yan H, Ohashi S, Gotoh Y, Sato A, Tsutsui H, Kaisho T, Toda T, Tsuji NM. Lactococcus lactis subsp. cremoris FC triggers IFN-γ production from NK and T cells via IL-12 and IL-18. Int Immunopharmacol. 2012;14(4):729–33.
Costabile A, Bergillos-Meca T, Rasinkangas P, Korpela K, de Vos WM, Gibson GR. Effects of soluble corn fiber alone or in synbiotic combination with lactobacillus rhamnosus gg and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: a randomized, double-blind, placebo-controlled, crossover study in healthy elderly (Saimes Study). Front Immunol. 2017;8:1443.
Qiu Y, Jiang Z, Hu S, Wang L, Ma X, Yang X. Lactobacillus plantarum Enhanced IL-22 Production in Natural Killer (NK) cells that protect the integrity of intestinal epithelial cell barrier damaged by enterotoxigenic Escherichia coli. Int J Mol Sci. 2017;18(11):2409.
Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2(5):328–39.
Sutherland DB, Suzuki K, Fagarasan S. Fostering of advanced mutualism with gut microbiota by immunoglobulin A. Immunol Rev. 2016;270(1):20–31.
Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129(6):729–39.
Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67(4):1992–2000.
Lundell AC, Björnsson V, Ljung A, Ceder M, Johansen S, Lindhagen G, Törnhage CJ, Adlerberth I, Wold AE, Rudin A. Infant B cell memory differentiation and early gut bacterial colonization. J Immunol. 2012;188(9):4315–22.
Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41(1):152–65.
Kim M, Kim CH. Regulation of humoral immunity by gut microbial products. Gut Microbes. 2017;8(4):392–9.
Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV, et al. Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342(6163):1243–6.
Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, Round JL. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe. 2015;17(2):153–63.
Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20(2):202–14.
Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33(1):71–83.
Chu VT, Beller A, Rausch S, Strandmark J, Zänker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40(4):582–93.
Kunisawa J, Hashimoto E, Inoue A, Nagasawa R, Suzuki Y, Ishikawa I, Shikata S, Arita M, Aoki J, Kiyono H. Regulation of intestinal IgA responses by dietary palmitic acid and its metabolism. J Immunol. 2014;193(4):1666–71.
Buchta CM, Bishop GA. Toll-like receptors and B cells: functions and mechanisms. Immunol Res. 2014;59(1–3):12–22.
Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med. 2014;20(11):1334–9.
St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30(9):695–704.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–16.
Yu AI, Zhao L, Eaton KA, Ho S, Chen J, Poe S, Becker J, Gonzalez A, McKinstry D, Hasso M, et al. Gut microbiota modulate CD8 T cell responses to influence colitis-associated tumorigenesis. Cell Rep. 2020;31(1):107471.
Shimokawa C, Kato T, Takeuchi T, Ohshima N, Furuki T, Ohtsu Y, Suzue K, Imai T, Obi S, Olia A, et al. CD8(+) regulatory T cells are critical in prevention of autoimmune-mediated diabetes. Nat Commun. 2020;11(1):1922.
Jaeger N, Gamini R, Cella M, Schettini JL, Bugatti M, Zhao S, Rosadini CV, Esaulova E, Di Luccia B, Kinnett B, et al. Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat Commun. 2021;12(1):1921.
Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, Adhikary T, Visekruna A. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 2018;8(1):14430.
Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dähling S, Kastenmüller W, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51(2):285-297.e285.
Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–89.
Renaude E, Kroemer M, Borg C, Peixoto P, Hervouet E, Loyon R, Adotévi O. Epigenetic reprogramming of CD4(+) helper T cells as a strategy to improve anticancer immunotherapy. Front Immunol. 2021;12:669992.
Hegazy AN, West NR, Stubbington MJT, Wendt E, Suijker KIM, Datsi A, This S, Danne C, Campion S, Duncan SH, et al. Circulating and tissue-resident CD4(+) T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology. 2017;153(5):1320-1337.e1316.
Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol Immunol. 2019;16(7):634–43.
Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12(5):793.
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66.
Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J Immunol. 2016;196(5):2388–400.
Lanz TV, Becker S, Mohapatra SR, Opitz CA, Wick W, Platten M. Suppression of Th1 differentiation by tryptophan supplementation in vivo. Amino Acids. 2017;49(7):1169–75.
Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, Radice E, Mariani A, Testoni PA, Canducci F, et al. High frequency of intestinal T(H)17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3(7):e1700492.
Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153(6):1621–33.
Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–67.
O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–102.
Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14(4):372–9.
Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci USA. 2015;112(22):7061–6.
Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun. 2018;87:38–49.
Hurtado CG, Wan F, Housseau F, Sears CL. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology. 2018;155(6):1706–15.
Lochner M, Bérard M, Sawa S, Hauer S, Gaboriau-Routhiau V, Fernandez TD, Snel J, Bousso P, Cerf-Bensussan N, Eberl G. Restricted microbiota and absence of cognate TCR antigen leads to an unbalanced generation of Th17 cells. J Immunol. 2011;186(3):1531–7.
Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85.
Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. Mucosal immunology. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015;349(6251):989–93.
Schnupf P, Gaboriau-Routhiau V, Sansonetti PJ, Cerf-Bensussan N. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr Opin Microbiol. 2017;35:100–9.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98.
Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163(2):367–80.
Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817–29.
Bellone M, Brevi A, Huber S. Microbiota-propelled T helper 17 cells in inflammatory diseases and cancer. Microbiol Mol Biol Rev. 2020;84(2):10–1128.
Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu HJ, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA. 2016;113(50):E8141-e8150.
Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455(7214):808–12.
Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature. 2019;576(7785):143–8.
Sun CY, Yang N, Zheng ZL, Liu D, Xu QL. T helper 17 (Th17) cell responses to the gut microbiota in human diseases. Biomed Pharmacother. 2023;161:114483.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551(7682):585–9.
Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, Turnbaugh JA, Verdin E, Hall KD, Leibel RL, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263-1275.e1216.
Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42.
Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity. 2014;41(5):789–801.
Jones L, Ho WQ, Ying S, Ramakrishna L, Srinivasan KG, Yurieva M, Ng WP, Subramaniam S, Hamadee NH, Joseph S, et al. A subpopulation of high IL-21-producing CD4(+) T cells in Peyer’s Patches is induced by the microbiota and regulates germinal centers. Sci Rep. 2016;6:30784.
Beller A, Kruglov A, Durek P, von Goetze V, Werner K, Heinz GA, Ninnemann J, Lehmann K, Maier R, Hoffmann U, et al. Specific microbiota enhances intestinal IgA levels by inducing TGF-β in T follicular helper cells of Peyer’s patches in mice. Eur J Immunol. 2020;50(6):783–94.
Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, Umesaki Y, Wu HJ. gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s Patch T follicular helper cells. Immunity. 2016;44(4):875–88.
Proietti M, Perruzza L, Scribano D, Pellegrini G, D’Antuono R, Strati F, Raffaelli M, Gonzalez SF, Thelen M, Hardt WD, et al. ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat Commun. 2019;10(1):250.
Wang L, Zhu L, Qin S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J Immunol Res. 2019;2019:4735040.
Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271(1):246–59.
Bates NA, Li A, Fan T, Cutcliffe MP, Dagenet CB, Sleiman KC, Ma H, Tahsin S, Garrett CS, Altemus J, et al. Gut commensal segmented filamentous bacteria fine-tune T follicular regulatory cells to modify the severity of systemic autoimmune arthritis. J Immunol. 2021;206(5):941–52.
Takahashi D, Hoshina N, Kabumoto Y, Maeda Y, Suzuki A, Tanabe H, Isobe J, Yamada T, Muroi K, Yanagisawa Y, et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine. 2020;58:102913.
Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–66.
Yan Y, Ramanan D, Rozenberg M, McGovern K, Rastelli D, Vijaykumar B, Yaghi O, Voisin T, Mosaheb M, Chiu I, et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity. 2021;54(3):499-513.e495.
Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, et al. Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature. 2020;577(7790):410–5.
Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016;16(5):295–309.
Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17(8):497–511.
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86.
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6.
Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107(27):12204–9.
Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352(6289):1116–20.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73.
Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, Hao S, Pokrovskii M, Xu M, Talbot J, et al. A RORγt(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. 2022;610(7933):737–43.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–8.
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22.
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8.
Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, Gough MJ, Urba WJ, Fox BA. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(20):6165–77.
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8.
Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12(1):92.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44.
Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(3):409–16.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, Wang N, Zhang C, Kong L, Liu Y, et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-Type colorectal cancer via metabolic pathway. Front Microbiol. 2020;11:814.
Pingili AK, Chaib M, Sipe LM, Miller EJ, Teng B, Sharma R, Yarbro JR, Asemota S, Al Abdallah Q, Mims TS, et al. Immune checkpoint blockade reprograms systemic immune landscape and tumor microenvironment in obesity-associated breast cancer. Cell Rep. 2021;35(12):109285.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8.
Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848–55.
Lee KA, Thomas AM, Bolte LA, Björk JR, de Ruijter LK, Armanini F, Asnicar F, Blanco-Miguez A, Board R, Calbet-Llopart N, et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28(3):535–44.
Wind TT, Gacesa R, Vich Vila A, de Haan JJ, Jalving M, Weersma RK, Hospers GAP. Gut microbial species and metabolic pathways associated with response to treatment with immune checkpoint inhibitors in metastatic melanoma. Melanoma Res. 2020;30(3):235–46.
McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM, Fernandes MR, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28(3):545–56.
Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2019;14(8):1378–89.
Newsome RC, Gharaibeh RZ, Pierce CM, da Silva WV, Paul S, Hogue SR, Yu Q, Antonia S, Conejo-Garcia JR, Robinson LA, et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022;14(1):35.
Katayama Y, Yamada T, Shimamoto T, Iwasaku M, Kaneko Y, Uchino J, Takayama K. The role of the gut microbiome on the efficacy of immune checkpoint inhibitors in Japanese responder patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):847–53.
Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, Terrisse S, Derosa L, Zitvogel L, Routy B, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. 2020;8(10):1243–50.
Song P, Yang D, Wang H, Cui X, Si X, Zhang X, Zhang L. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac Cancer. 2020;11(6):1621–32.
Vernocchi P, Gili T, Conte F, Del Chierico F, Conta G, Miccheli A, Botticelli A, Paci P, Caldarelli G, Nuti M, et al. Network analysis of gut microbiome and metabolome to discover microbiota-linked biomarkers in patients affected by non-small cell lung cancer. Int J Mol Sci. 2020;21(22):8730.
Zhang C, Wang J, Sun Z, Cao Y, Mu Z, Ji X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci. 2021;112(8):3005–17.
Lee SH, Cho SY, Yoon Y, Park C, Sohn J, Jeong JJ, Jeon BN, Jang M, An C, Lee S, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277–88.
Grenda A, Iwan E, Chmielewska I, Krawczyk P, Giza A, Bomba A, Frąk M, Rolska A, Szczyrek M, Kieszko R, et al. Presence of Akkermansiaceae in gut microbiome and immunotherapy effectiveness in patients with advanced non-small cell lung cancer. AMB Express. 2022;12(1):86.
Fang C, Fang W, Xu L, Gao F, Hou Y, Zou H, Ma Y, Moll JM, Yang Y, Wang D, et al. Distinct functional metagenomic markers predict the responsiveness to anti-PD-1 therapy in chinese non-small cell lung cancer patients. Front Oncol. 2022;12:837525.
Yin H, Yang L, Peng G, Yang K, Mi Y, Hu X, Hao X, Jiao Y, Wang X, Wang Y. The commensal consortium of the gut microbiome is associated with favorable responses to anti-programmed death protein 1 (PD-1) therapy in thoracic neoplasms. Cancer Biol Med. 2021;18(4):1040–52.
Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):193.
Mao J, Wang D, Long J, Yang X, Lin J, Song Y, Xie F, Xun Z, Wang Y, Wang Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer 2021;9(12):e003334.
Li L, Ye J. Characterization of gut microbiota in patients with primary hepatocellular carcinoma received immune checkpoint inhibitors: a Chinese population-based study. Medicine. 2020;99(37):e21788.
Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW, Cho SB, Joo YE, Hwang JE, Bae WK, Chung IJ, et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J Gastroenterol. 2021;27(42):7340–9.
Ponziani FR, De Luca A, Picca A, Marzetti E, Petito V, Del Chierico F, Reddel S, Paroni Sterbini F, Sanguinetti M, Putignani L, et al. Gut dysbiosis and fecal calprotectin predict response to immune checkpoint inhibitors in patients with hepatocellular carcinoma. Hepatol Commun. 2022;6:1492–501.
Shen YC, Lee PC, Kuo YL, Wu WK, Chen CC, Lei CH, Yeh CP, Hsu C, Hsu CH, Lin ZZ, et al. An exploratory study for the association of gut microbiome with efficacy of immune checkpoint inhibitor in patients with hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:809–22.
Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020;78(2):195–206.
Salgia NJ, Bergerot PG, Maia MC, Dizman N, Hsu J, Gillece JD, Folkerts M, Reining L, Trent J, Highlander SK, et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol. 2020;78(4):498–502.
Vétizou M, Bi D, Xie R, Li M, Guo J, Liu H, Guo X, Fang J, Ding T, Zhu H, et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct Target Ther. 2021;6(1):398.
Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110(8):3047–52.
Moyat M, Velin D. Immune responses to Helicobacter pylori infection. World J Gastroenterol. 2014;20(19):5583–93.
Oster P, Vaillant L, Riva E, McMillan B, Begka C, Truntzer C, Richard C, Leblond MM, Messaoudene M, Machremi E, et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2022;71(3):457–66.
Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282(5397):2263–6.
Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164(10):5319–27.
Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA. 2002;99(18):11790–5.
Hoff H, Kolar P, Ambach A, Radbruch A, Brunner-Weinzierl MC. CTLA-4 (CD152) inhibits T cell function by activating the ubiquitin ligase Itch. Mol Immunol. 2010;47(10):1875–81.
Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–3.
Romo-Tena J, Gómez-Martín D, Alcocer-Varela J. CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmun Rev. 2013;12(12):1171–6.
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5.
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6.
Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Can Res. 1997;57(18):4036–41.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–57.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105(39):14987–92.
Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci USA. 2009;106(8):2729–34.
Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205(9):2125–38.
Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–25.
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Pitt JM, Vétizou M, Gomperts Boneca I, Lepage P, Chamaillard M, Zitvogel L. Enhancing the clinical coverage and anticancer efficacy of immune checkpoint blockade through manipulation of the gut microbiota. Oncoimmunology. 2017;6(1):e1132137.
Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(6):1368–79.
Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix L, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11(1):2168.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
Met Ö, Jensen KM, Chamberlain CA, Donia M, Svane IM. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019;41(1):49–58.
Merhavi-Shoham E, Itzhaki O, Markel G, Schachter J, Besser MJ. Adoptive cell therapy for metastatic melanoma. Cancer J. 2017;23(1):48–53.
Zhang R, Zhang Z, Liu Z, Wei D, Wu X, Bian H, Chen Z. Adoptive cell transfer therapy for hepatocellular carcinoma. Front Med. 2019;13(1):3–11.
Fan J, Shang D, Han B, Song J, Chen H, Yang JM. Adoptive cell transfer: Is it a promising immunotherapy for colorectal cancer? Theranostics. 2018;8(20):5784–800.
Holstein SA, Lunning MA. CAR T-Cell therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107(1):112–22.
Yu S, Li A, Liu Q, Li T, Yuan X, Han X, Wu K. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78.
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Sciencey. 2015;348(6230):62–8.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
Abid MB, Shah NN, Maatman TC, Hari PN. Gut microbiome and CAR-T therapy. Exp Hematol Oncol. 2019;8:31.
Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Investig. 2007;117(8):2197–204.
Uribe-Herranz M, Bittinger K, Rafail S, Guedan S, Pierini S, Tanes C, Ganetsky A, Morgan MA, Gill S, Tanyi JL, et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12. JCI Insight 2018;3(4):e94952.
Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, Bruno TC, Vignali DAA, Hand TW. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. 2021;54(12):2812-2824.e2814.
Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, Slingerland JB, Beghi S, Herrera PS, Giardina P, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28(4):713–23.
Schubert ML, Rohrbach R, Schmitt M, Stein-Thoeringer CK. The potential role of the intestinal micromilieu and individual microbes in the immunobiology of chimeric antigen receptor T-cell therapy. Front Immunol. 2021;12:670286.
Hanagata N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int J Nanomed. 2017;12:515–31.
Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4(4):249–58.
Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Tötterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33(3):225–35.
Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, Guiducci C. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci USA. 2016;113(46):E7240-e7249.
Voeller J, Erbe AK, Slowinski J, Rasmussen K, Carlson PM, Hoefges A, VandenHeuvel S, Stuckwisch A, Wang X, Gillies SD, et al. Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition. J Immunother Cancer. 2019;7(1):344.
Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19(2):77–94.
Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602.
Carretta MD, Quiroga J, López R, Hidalgo MA, Burgos RA. Participation of short-chain fatty acids and their receptors in gut inflammation and colon cancer. Front Physiol. 2021;12:662739.
Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7 Suppl):2485s–93s.
Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Can Res. 2009;69(7):2826–32.
Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285(36):27601–8.
Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9(1):3555.
Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, Fosso B, Melocchi L, Nizzoli G, Troisi J, et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol. 2020;5(3):511–24.
Santoni M, Piva F, Conti A, Santoni A, Cimadamore A, Scarpelli M, Battelli N, Montironi R. Re: gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Eur Urol. 2018;74(4):521–2.
Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132(5):1012–7.
Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. 2002;130(2):245–55.
Liu L, Li L, Min J, Wang J, Wu H, Zeng Y, Chen S, Chu Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277(1–2):66–73.
Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13(11):1299–307.
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39.
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, Dong X, Huang J, Wang Q, Mackay CR, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33(5):988-1000.e1007.
Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, Klein M, Wempe A, Leister H, Raifer H, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077.
Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ. Dietary fiber confers protection against flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity. 2018;48(5):992-1005.e1008.
Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106(10):3698–703.
Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–40.
Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32.
Botticelli A, Cerbelli B, Lionetto L, Zizzari I, Salati M, Pisano A, Federica M, Simmaco M, Nuti M, Marchetti P. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J Transl Med. 2018;16(1):219.
Kocher F, Amann A, Zimmer K, Geisler S, Fuchs D, Pichler R, Wolf D, Kurz K, Seeber A, Pircher A. High indoleamine-2,3-dioxygenase 1 (IDO) activity is linked to primary resistance to immunotherapy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2021;10(1):304–13.
Li H, Bullock K, Gurjao C, Braun D, Shukla SA, Bossé D, Lalani AA, Gopal S, Jin C, Horak C, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. 2019;10(1):4346.
Karayama M, Masuda J, Mori K, Yasui H, Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Nakamura Y, et al. Comprehensive assessment of multiple tryptophan metabolites as potential biomarkers for immune checkpoint inhibitors in patients with non-small cell lung cancer. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst MEXICO. 2021;23(2):418–23.
Hezaveh K, Shinde RS, Klötgen A, Halaby MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55(2):324-340.e328.
Renga G, Nunzi E, Pariano M, Puccetti M, Bellet MM, Pieraccini G, D'Onofrio F, Santarelli I, Stincardini C, Aversa F, et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J Immunother Cancer 2022;10(3):e003725.
Almonte AA, Rangarajan H, Yip D, Fahrer AM. How does the gut microbiome influence immune checkpoint blockade therapy? Immunol Cell Biol. 2021;99(4):361–72.
Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2020;117(32):19376–87.
Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J, Jiang Z, Jiang Z, Hsiao WW, Liu H, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71(4):734–45.
Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2022;21:236–47.
Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, Yu-Lun K, Chou SH, Luo JC, Hou MC, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer 2022;10(6):e004779.
Goossens JF, Bailly C. Ursodeoxycholic acid and cancer: from chemoprevention to chemotherapy. Pharmacol Ther. 2019;203:107396.
Krishna-Subramanian S, Hanski ML, Loddenkemper C, Choudhary B, Pagès G, Zeitz M, Hanski C. UDCA slows down intestinal cell proliferation by inducing high and sustained ERK phosphorylation. Int J Cancer. 2012;130(12):2771–82.
Shen Y, Lu C, Song Z, Qiao C, Wang J, Chen J, Zhang C, Zeng X, Ma Z, Chen T, et al. Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β degradation. Nat Commun. 2022;13(1):3419.
Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391):eaan5931.
Wu J, Gong J, Geng J, Song Y. Deoxycholic acid induces the overexpression of intestinal mucin, MUC2, via NF-kB signaling pathway in human esophageal adenocarcinoma cells. BMC Cancer. 2008;8:333.
Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, Wang JY, Cheng E, Meyer F, Wang DH, et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κB activation in benign Barrett’s epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G278-286.
Centuori SM, Martinez JD. Differential regulation of EGFR-MAPK signaling by deoxycholic acid (DCA) and ursodeoxycholic acid (UDCA) in colon cancer. Dig Dis Sci. 2014;59(10):2367–80.
Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014;12:164.
Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–9.
Lasek W, Janyst M, Wolny R, Zapała Ł, Bocian K, Drela N. Immunomodulatory effects of inosine pranobex on cytokine production by human lymphocytes. Acta Pharm. 2015;65(2):171–80.
Shinohara Y, Tsukimoto M. Guanine and inosine nucleotides/nucleosides suppress murine T cell activation. Biochem Biophys Res Commun. 2018;498(4):764–8.
Csóka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Németh ZH, Haskó G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22(10):3491–9.
He B, Hoang TK, Wang T, Ferris M, Taylor CM, Tian X, Luo M, Tran DQ, Zhou J, Tatevian N, et al. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med. 2017;214(1):107–23.
Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Can Res. 2014;74(24):7239–49.
Wang T, Liu G, Wang R. The intercellular metabolic interplay between tumor and immune cells. Front Immunol. 2014;5:358.
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41.
DeNicola GM, Cantley LC. Cancer’s fuel choice: new flavors for a picky eater. Mol Cell. 2015;60(4):514–23.
Wang T, Gnanaprakasam JNR, Chen X, Kang S, Xu X, Sun H, Liu L, Rodgers H, Miller E, Cassel TA, et al. Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat Metab. 2020;2(7):635–47.
Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, Fanger GR, Hang HC. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–6.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–92.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68.
Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol. 2018;9(9):180.
Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11(2):91–9.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85.
Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe’er D, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and Anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120-1133.e1117.
Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, Li X, Zhu H, Zhong X, Pan J, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer. 2019;19(1):559.
Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391.
Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA. 2018;115(1):157–61.
Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, Lv Z, Liang Q, Fischbach MA, Sonnenburg JL, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci USA. 2020;117(44):27509–15.
Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804–8.
Wang T, Zheng N, Luo Q, Jiang L, He B, Yuan X, Shen L. Probiotics lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front Immunol. 2019;10:1235.
Liu T, Xiong Q, Li L, Hu Y. Intestinal microbiota predicts lung cancer patients at risk of immune-related diarrhea. Immunotherapy. 2019;11(5):385–96.
Tan B, Chen MJ, Guo Q, Tang H, Li Y, Jia XM, Xu Y, Zhu L, Wang MZ, Qian JM. Clinical-radiological characteristics and intestinal microbiota in patients with pancreatic immune-related adverse events. Thorac Cancer. 2021;12(12):1814–23.
Liu W, Ma F, Sun B, Liu Y, Tang H, Luo J, Chen H, Luo Z. Intestinal microbiome associated with immune-related adverse events for patients treated with anti-PD-1 inhibitors, a real-world study. Front Immunol. 2021;12:756872.
Usyk M, Pandey A, Hayes RB, Moran U, Pavlick A, Osman I, Weber JS, Ahn J. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 2021;13(1):160.
Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145(5):946–53.
Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer. 2019;145(8):2021–31.
Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9(2):88–96.
Shaikh FY, Gills JJ, Mohammad F, White JR, Stevens CM, Ding H, Fu J, Tam A, Blosser RL, Domingue JC, et al. Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors. Cancer Immunol Immunother. 2022;71:2405–20.
Routy B, Lenehan JG, Miller WH Jr, Jamal R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L, Puncochar M, et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2023;29(8):2121–32.
Fillon M. Fecal microbiota transplants may aid melanoma immunotherapy resistance. CA Cancer J Clin. 2021;71(4):285–6.
Spreafico A, Heirali AA, Araujo DV, Tan TJ, Oliva M, Schneeberger PHH, Chen B, Wong MK, Stayner LA, Hansen AR, et al. First-in-class microbial ecosystem therapeutic 4 (MET4) in combination with immune checkpoint inhibitors in patients with advanced solid tumors (MET4-IO trial). Ann Oncol. 2023;34(6):520–30.
Khanna S, Assi M, Lee C, Yoho D, Louie T, Knapple W, Aguilar H, Garcia-Diaz J, Wang GP, Berry SM, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent clostridioides difficile infection. Drugs. 2022;82(15):1527–38.
Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med. 2022;386(3):220–9.
DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–50.
Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108.
Baruch EN, Gaglani T, Wargo JA. Fecal microbiota transplantation as a mean of overcoming immunotherapy-resistant cancers - Hype or hope? Ther Adv Med Oncol. 2021;13:17588359211045852.
McQuade JL, Ologun GO, Arora R, Wargo JA. Gut microbiome modulation via fecal microbiota transplant to augment immunotherapy in patients with melanoma or other cancers. Curr Oncol Rep. 2020;22(7):74.
Ianiro G, Punčochář M, Karcher N, Porcari S, Armanini F, Asnicar F, Beghini F, Blanco-Míguez A, Cumbo F, Manghi P, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. 2022;28(9):1913–23.
Klement RJ, Pazienza V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina. 2019;55(4):84.
Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838.
Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. 2020;20(2):125–38.
Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8):2795.
Matijasic BB, Obermajer T, Lipoglavsek L, Grabnar I, Avgustin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014;53(4):1051–64.
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Bibbò S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, Cammarota G. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20(22):4742–9.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73.
Russo E, Nannini G, Dinu M, Pagliai G, Sofi F, Amedei A. Exploring the food-gut axis in immunotherapy response of cancer patients. World J Gastroenterol. 2020;26(33):4919–32.
Szczyrek M, Bitkowska P, Chunowski P, Czuchryta P, Krawczyk P, Milanowski J. Diet, microbiome, and cancer immunotherapy-a comprehensive review. Nutrients. 2021;13(7):2217.
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–40.
Woolf EC, Syed N, Scheck AC. Tumor metabolism, the ketogenic diet and β-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122.
Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95.
Sremanakova J, Sowerbutts AM, Burden S. A systematic review of the use of ketogenic diets in adult patients with cancer. J Human Nutr Diet. 2018;31(6):793–802.
Ferrere G, Tidjani Alou M, Liu P, Goubet AG, Fidelle M, Kepp O, Durand S, Iebba V, Fluckiger A, Daillère R, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 2021;6(2):e145207.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14.
Gamallat Y, Meyiah A, Kuugbee ED, Hago AM, Chiwala G, Awadasseid A, Bamba D, Zhang X, Shang X, Luo F, et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed Pharmacother. 2016;83:536–41.
Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán LG. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol. 2017;8:1553.
Kaźmierczak-Siedlecka K, Roviello G, Catalano M, Polom K. Gut microbiota modulation in the context of immune-related aspects of lactobacillus spp. and bifidobacterium spp. in gastrointestinal cancers. Nutrients. 2021;13(8):2674.
Wan L, Wu C, Wu Q, Luo S, Liu J, Xie X. Impact of probiotics use on clinical outcomes of immune checkpoint inhibitors therapy in cancer patients. Cancer Med. 2022;12:1841–9.
Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28(4):704–12.
Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S, et al. Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. 2020;8(10):1236–42.
Gao G, Ma T, Zhang T, Jin H, Li Y, Kwok LY, Zhang H, Sun Z. Adjunctive probiotic lactobacillus rhamnosus probio-m9 administration enhances the effect of Anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol. 2021;12:772532.
Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, Okamoto T, Hamatake M, Tsuchiya-Kawano Y, Otsubo K, et al. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: a multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int J Cancer. 2021;149(2):473–82.
Zhuo Q, Yu B, Zhou J, Zhang J, Zhang R, Xie J, Wang Q, Zhao S. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep. 2019;9(1):20128.
Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–5.
Montalban-Arques A, Katkeviciute E, Busenhart P, Bircher A, Wirbel J, Zeller G, Morsy Y, Borsig L, Glaus Garzon JF, Müller A, et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe. 2021;29(10):1573-1588.e1577.
Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–29.
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-1423.e1416.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502.
Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol. 2019;103(16):6463–72.
Liu X, Wang L, Jing N, Jiang G, Liu Z. Biostimulating gut microbiome with bilberry anthocyanin combo to enhance anti-PD-L1 efficiency against murine colon cancer. Microorganisms. 2020;8(2):175.
Wang L, Jiang G, Jing N, Liu X, Li Q, Liang W, Liu Z. Bilberry anthocyanin extracts enhance anti-PD-L1 efficiency by modulating gut microbiota. Food Funct. 2020;11(4):3180–90.
Zhang SL, Mao YQ, Zhang ZY, Li ZM, Kong CY, Chen HL, Cai PR, Han B, Ye T, Wang LS. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics. 2021;11(9):4155–70.
Han K, Nam J, Xu J, Sun X, Huang X, Animasahun O, Achreja A, Jeon JH, Pursley B, Kamada N, et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat Biomed Eng. 2021;5(11):1377–88.
Park HJ, Boo S, Park I, Shin MS, Takahashi T, Takanari J, Homma K, Kang I. AHCC(®), a standardized extract of cultured lentinula edodes mycelia, promotes the anti-tumor effect of dual immune checkpoint blockade effect in murine colon cancer. Front Immunol. 2022;13:875872.
Messaoudene M, Pidgeon R, Richard C, Ponce M, Diop K, Benlaifaoui M, Nolin-Lapalme A, Cauchois F, Malo J, Belkaid W, et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 2022;12(4):1070–87.
Charbonneau MR, Isabella VM, Li N, Kurtz CB. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat Commun. 2020;11(1):1738.
Wei C, Xun AY, Wei XX, Yao J, Wang JY, Shi RY, Yang GH, Li YX, Xu ZL, Lai MG, et al. Bifidobacteria expressing tumstatin protein for antitumor therapy in tumor-bearing mice. Technol Cancer Res Treat. 2016;15(3):498–508.
Din MO, Danino T, Prindle A, Skalak M, Selimkhanov J, Allen K, Julio E, Atolia E, Tsimring LS, Bhatia SN, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 2016;536(7614):81–5.
Liang K, Liu Q, Li P, Luo H, Wang H, Kong Q. Genetically engineered salmonella typhimurium: recent advances in cancer therapy. Cancer Lett. 2019;448:168–81.
Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019;25(7):1057–63.
Leventhal DS, Sokolovska A, Li N, Plescia C, Kolodziej SA, Gallant CW, Christmas R, Gao JR, James MJ, Abin-Fuentes A, et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat Commun. 2020;11(1):2739.
Binder DC, Engels B, Arina A, Yu P, Slauch JM, Fu YX, Karrison T, Burnette B, Idel C, Zhao M, et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res. 2013;1(2):123–33.
Mkrtichyan M, Chong N, Abu Eid R, Wallecha A, Singh R, Rothman J, Khleif SN. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15.
Canale FP, Basso C, Antonini G, Perotti M, Li N, Sokolovska A, Neumann J, James MJ, Geiger S, Jin W, et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598(7882):662–6.
Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18(12):727–43.
Federici S, Nobs SP, Elinav E. Phages and their potential to modulate the microbiome and immunity. Cell Mol Immunol. 2021;18(4):889–904.
Zheng DW, Dong X, Pan P, Chen KW, Fan JX, Cheng SX, Zhang XZ. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717–28.
Dong X, Pan P, Zheng DW, Bao P, Zeng X, Zhang XZ. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6(20):eaba1590.
Zuo T, Wong SH, Lam K, Lui R, Cheung K, Tang W, Ching JYL, Chan PKS, Chan MCW, Wu JCY, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2018;67(4):634–43.
Draper LA, Ryan FJ, Smith MK, Jalanka J, Mattila E, Arkkila PA, Ross RP, Satokari R, Hill C. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6(1):220.
Sinha R, Abu-Ali G, Vogtmann E, Fodor AA, Ren B, Amir A, Schwager E, Crabtree J, Ma S, Abnet CC, et al. Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat Biotechnol. 2017;35(11):1077–86.
Faust K, Lahti L, Gonze D, de Vos WM, Raes J. Metagenomics meets time series analysis: unraveling microbial community dynamics. Curr Opin Microbiol. 2015;25:56–66.
Zhao LY, Song J, Liu Y, Song CX, Yi C. Mapping the epigenetic modifications of DNA and RNA. Protein Cell. 2020;11(11):792–808.
Hu J, Amor DR, Barbier M, Bunin G, Gore J. Emergent phases of ecological diversity and dynamics mapped in microcosms. Science. 2022;378(6615):85–9.
Nguyen CL, Markey KA, Miltiadous O, Dai A, Waters N, Sadeghi K, Fei T, Shouval R, Taylor BP, Liao C, et al. High-resolution analyses of associations between medications, microbiome, and mortality in cancer patients. Cell. 2023;186(12):2705–18.
Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell. 2021;39(10):1317–41.
Shi H, Shi Q, Grodner B, Lenz JS, Zipfel WR, Brito IL, De Vlaminck I. Highly multiplexed spatial mapping of microbial communities. Nature. 2020;588(7839):676–81.
Lloréns-Rico V, Simcock JA, Huys GRB, Raes J. Single-cell approaches in human microbiome research. Cell. 2022;185(15):2725–38.
Venturelli OS, Carr AC, Fisher G, Hsu RH, Lau R, Bowen BP, Hromada S, Northen T, Arkin AP. Deciphering microbial interactions in synthetic human gut microbiome communities. Mol Syst Biol. 2018;14(6):e8157.
Tan Y, Shen J, Si T, Ho CL, Li Y, Dai L. Engineered live biotherapeutics: progress and challenges. Biotechnol J. 2020;15(10):e2000155.
Cheng AG, Ho PY, Aranda-Díaz A, Jain S, Yu FB, Meng X, Wang M, Iakiviak M, Nagashima K, Zhao A, et al. Design, construction, and in vivo augmentation of a complex gut microbiome. Cell. 2022;185(19):3617-3636.e3619.
Marsh JW, Ley RE. Microbiome engineering: taming the untractable. Cell. 2022;185(3):416–8.
Zeng T, Yu X, Chen Z. Applying artificial intelligence in the microbiome for gastrointestinal diseases: a review. J Gastroenterol Hepatol. 2021;36(4):832–40.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China, No.82102998. The views expressed are those of the authors and not necessarily those of the NSF. We apologize for not being able to cite all the publications related to this topic due to space constraints of the journal.
Author information
Authors and Affiliations
Contributions
ZL, WX and ZL contributed equally to this work; the conception and design of the study: LZ, ZL; acquisition of data from published papers: ZL, JW, ZZ, DK, XL, XX, DZ; analysis and interpretation of data: ZL, WX, LZ; manuscript preparation and manuscript editing: ZL, WX, ZL; manuscript review and corresponding author: L.Z.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All auhors have read and approved the final manuscript.
Competing interests
The authors declares that they no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional files of studies and trials related to the gut microbiome.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Xiong, W., Liang, Z. et al. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J Hematol Oncol 17, 33 (2024). https://doi.org/10.1186/s13045-024-01541-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-024-01541-w